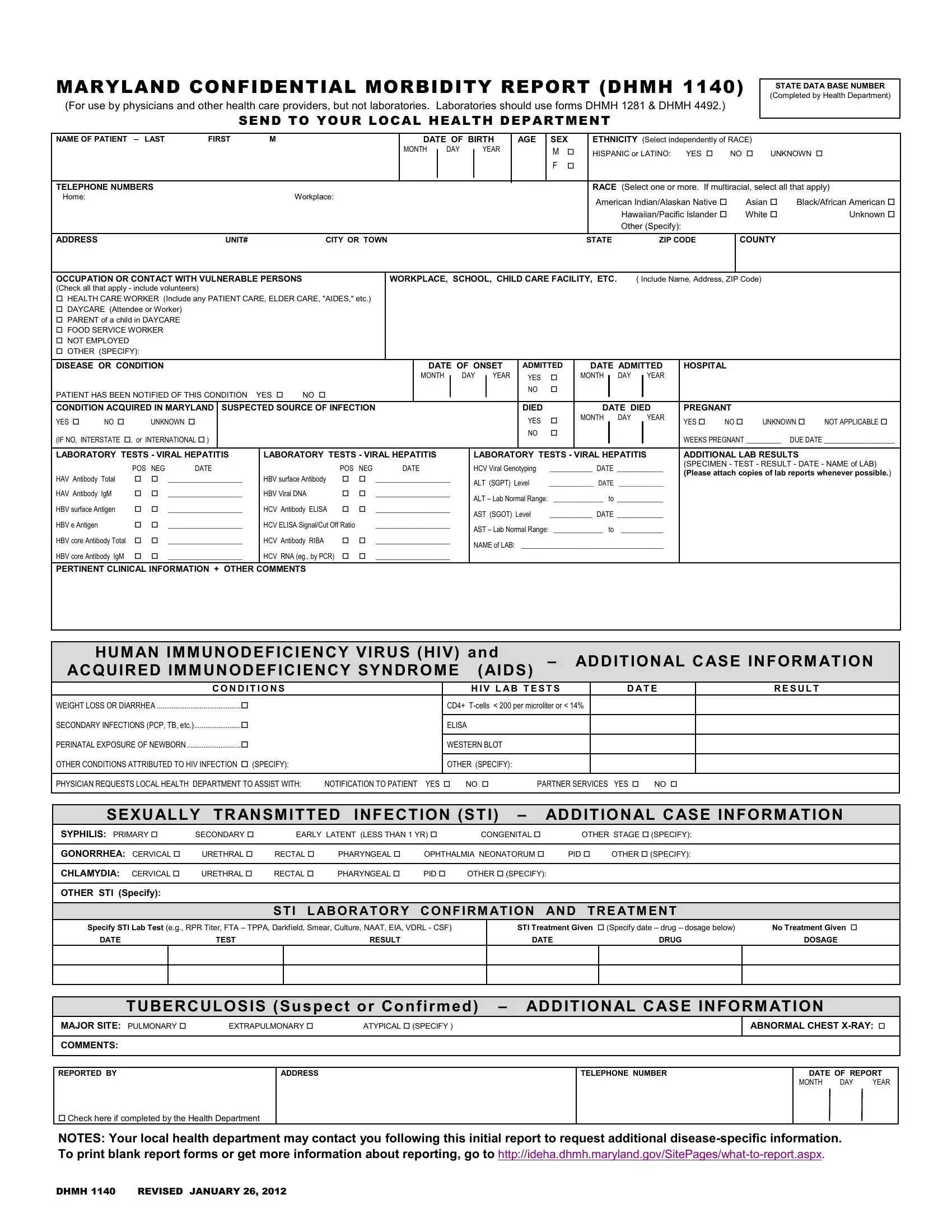
Through the online PDF editor by FormsPal, it is easy to fill out or alter HIV right here. To maintain our editor on the cutting edge of practicality, we aim to adopt user-driven capabilities and enhancements on a regular basis. We are routinely thankful for any suggestions - play a vital role in revolutionizing how we work with PDF documents. Here is what you'd need to do to get started:
Step 1: First, open the pdf tool by pressing the "Get Form Button" at the top of this site.
Step 2: Using this state-of-the-art PDF editor, you can do more than simply complete forms. Try each of the functions and make your forms appear perfect with customized text incorporated, or fine-tune the file's original content to excellence - all comes with the capability to incorporate stunning pictures and sign it off.
This form will need specific details; to guarantee consistency, be sure to take note of the guidelines down below:
1. When submitting the HIV, ensure to incorporate all necessary fields in their relevant area. This will help to speed up the work, enabling your details to be processed promptly and properly.

Step 3: Look through the details you have entered into the blank fields and click the "Done" button. After starting afree trial account at FormsPal, you'll be able to download HIV or email it without delay. The PDF will also be easily accessible through your personal account page with your modifications. FormsPal is devoted to the confidentiality of our users; we make certain that all information handled by our editor is confidential.