You are able to complete biomet 3i complaint form without difficulty with our PDFinity® online PDF tool. Our tool is constantly evolving to provide the very best user experience attainable, and that is because of our dedication to constant development and listening closely to feedback from users. Starting is effortless! All you need to do is adhere to the following simple steps below:
Step 1: Click the "Get Form" button above. It will open up our pdf tool so that you could begin filling in your form.
Step 2: With this online PDF editor, you may do more than merely fill out blank form fields. Edit away and make your forms seem high-quality with customized text incorporated, or adjust the file's original content to excellence - all accompanied by the capability to insert any kind of graphics and sign the document off.
This document requires specific info to be filled in, therefore ensure you take the time to type in what is expected:
1. Fill out the biomet 3i complaint form with a selection of necessary blank fields. Consider all the information you need and be sure there is nothing forgotten!
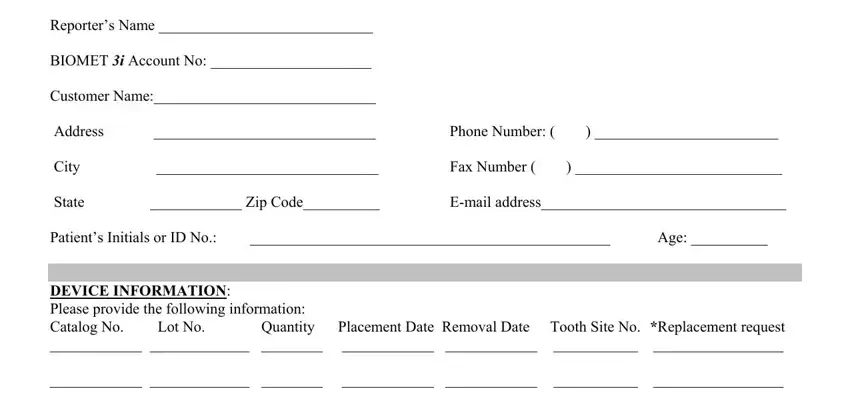
2. Your next part would be to fill in these blanks: Warranty Form To comply with, NonIntegration Loss of, If this event involved an implant, M Rev , and Restored.

It is possible to make an error while completing the If this event involved an implant, consequently make sure to take another look before you'll send it in.
3. This next segment is focused on For Implants only Did the patient, Smoker, Osteoporosis, Diabetes, Other, Yes, No, No, High density Type I, Xenograft Alloplast, Hybrid, Single Stage transgingival Two, No Yes describe material below, Autogenous Allograft, and Moderate density Type II Low - fill in every one of these blank fields.
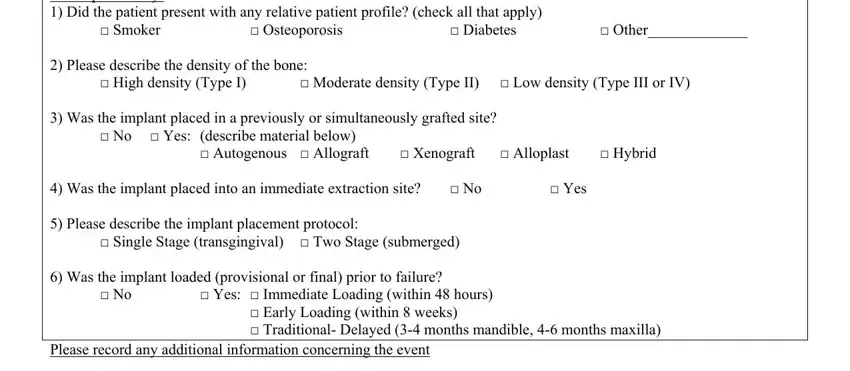
4. Filling in Please describe the density of, Date, Send To, BIOMET i, Regulatory ServicesImplant Warranty, Riverside Drive, Palm Beach Gardens FL , and Phone Fax is crucial in this section - always be patient and be mindful with each field!

Step 3: Right after going through the fields and details, click "Done" and you are good to go! Join FormsPal now and easily get access to biomet 3i complaint form, available for download. All alterations made by you are preserved , meaning you can edit the document later on if needed. Here at FormsPal.com, we do our utmost to make certain that all of your details are stored private.



