Completing cdph lab101 is simple. Our experts created our editor to make it simple to use and assist you to fill in any PDF online. Below are a few steps that you need to adhere to:
Step 1: You should press the orange "Get Form Now" button at the top of the website page.
Step 2: Now you will be on your form edit page. You'll be able to add, update, highlight, check, cross, add or erase areas or text.
Feel free to provide the next information to prepare the cdph lab101 PDF:

In the Marina Bay Parkway Richmond CA, ame, License Number, Telephone Day, Telephone Home, Mailing Address Number Street, City, tate, Zip Code, Please check this box if you have, INSTRUCTIONS Complete Section for, You must sign the sign, bottom of this form, ature line at the, and to certify th box, put in writing your information.
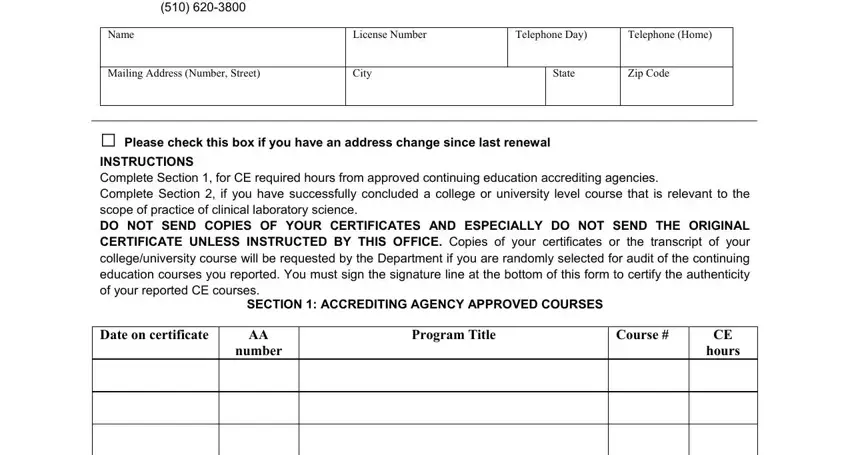
Type in the necessary data when you find yourself on the SECTION COLLEGE OR UNIVERSITY, CollegeU, nive, rsity, Course Title, SemesterQuarter units, Course Date, Have you been convicted of any, years Yes No Birth date mmddyy, I certify that I have taken the, for the courses from an accredited, Signature, Date, and LAB Page of pages section.
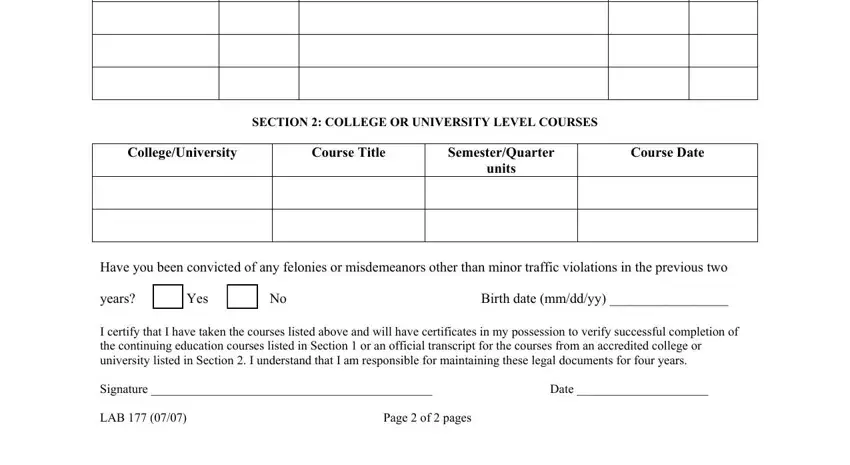
Step 3: After you hit the Done button, your prepared form can be easily exported to any kind of your gadgets or to electronic mail specified by you.
Step 4: Generate a copy of any file. It can save you some time and enable you to stay clear of difficulties as time goes on. Keep in mind, your information is not used or analyzed by us.