chemistry counting atoms worksheet can be filled out online effortlessly. Simply use FormsPal PDF tool to complete the task right away. The tool is constantly maintained by our staff, getting additional features and growing to be better. Starting is effortless! Everything you should do is adhere to these simple steps directly below:
Step 1: First of all, access the tool by clicking the "Get Form Button" in the top section of this webpage.
Step 2: With this advanced PDF editor, you can actually accomplish more than just complete forms. Edit away and make your forms appear perfect with customized text incorporated, or fine-tune the file's original content to perfection - all comes along with an ability to add your own pictures and sign the PDF off.
To be able to finalize this document, ensure that you provide the required details in each blank field:
1. When submitting the chemistry counting atoms worksheet, be certain to complete all necessary blank fields in its corresponding area. It will help hasten the process, allowing your details to be processed without delay and appropriately.
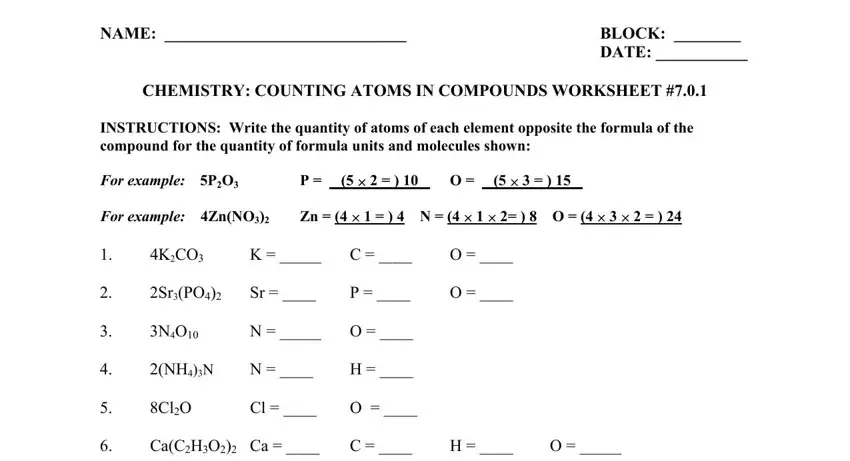
2. Immediately after this section is filled out, go to enter the applicable details in these - NaBr, AlOH, INSTRUCTIONS Write the quantity of, BaMnO Ba Mn O, AlSiO Al, GaCrO Ga, Si O, NaHCO, FeO, KNO, and MgSO.
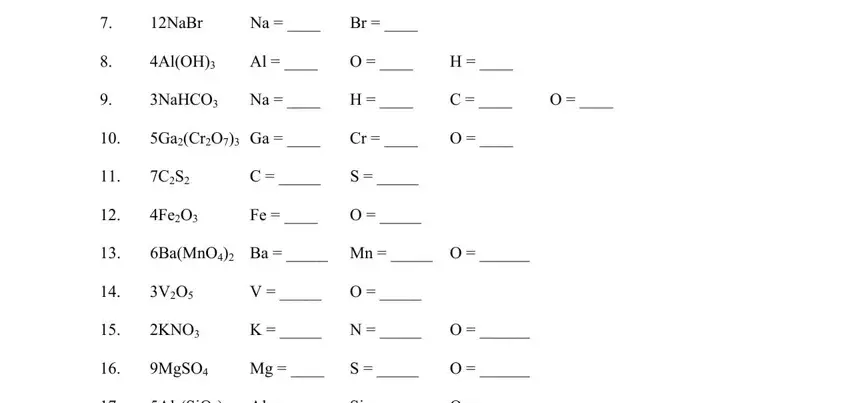
3. The third stage is normally easy - fill in all of the fields in INSTRUCTIONS Write the quantity of, AlSiO Al, I O, Si O, Continued, and AuIO to conclude this segment.
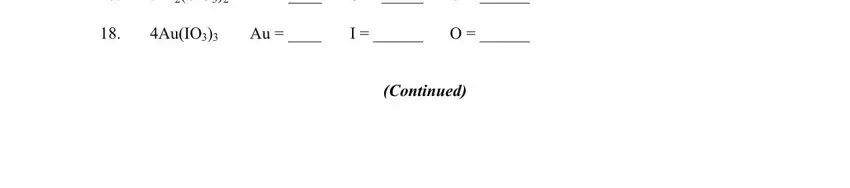
4. It is time to fill out the next section! In this case you've got all these SnCl, AsBr, SBr, HSO, CuSeO, S O, Se O, O P O, CaOH MgPO Mg, INSTRUCTIONS Write the quantity of, AlCHO Al, C H, ClF, H O, and KrCl form blanks to do.
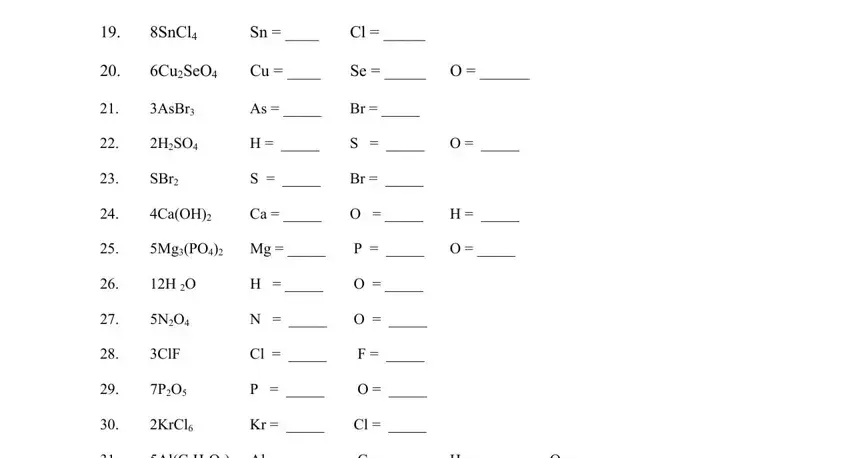
It is easy to make an error while filling in your C H, therefore make sure to reread it prior to when you send it in.
5. To finish your document, this last section has a number of additional blank fields. Filling in INSTRUCTIONS Write the quantity of, CuHSO Cu, BaCHO Ba, AlCHO Al, S O, NHCrO N, Mn O, C H, C O, SrMnO, NHNO, KZnO, AuNO, FePO, and AlCO should finalize everything and you can be done in a flash!

Step 3: Ensure your information is accurate and then press "Done" to conclude the project. After setting up afree trial account at FormsPal, you'll be able to download chemistry counting atoms worksheet or email it right off. The PDF document will also be easily accessible in your personal account with all your adjustments. With FormsPal, you can fill out forms without the need to get worried about personal information breaches or records getting distributed. Our protected software helps to ensure that your personal data is maintained safe.

