The FDA 2579 form, officially known as the "Report of Assembly of a Diagnostic X-Ray System," plays a crucial role in ensuring the safety and compliance of diagnostic x-ray equipment throughout the United States. Mandated by the Department of Health and Human Services and the Food and Drug Administration, this form is a necessary component of the regulatory framework that oversees the assembly, installation, and proper functioning of x-ray systems. Its comprehensive nature requires detailed information, including the location where the equipment is installed, assembler's details, general information on the system's intended use, component specifics, and certification by the assembler that all parts meet the diagnostic x-ray performance standards as set out in 21 CFR Part 1020. The form also documents the types of systems assembled, whether they are fully certified systems, reassemblies, replacements, or additions, and covers a broad spectrum of medical applications from general radiology to more specialized fields such as mammography and dental cephalometry. An assembler's affirmation signifies that all components were adjusted, tested, not modified adversely, and installed according to federal standards, ensuring that each x-ray system assembled contributes to safe and effective medical diagnostics. This rigorous documentation process, underscored by the FDA 2579 form, epitomizes the extensive measures taken to protect public health by ensuring diagnostic x-ray equipment adheres to the highest safety standards.
| Question | Answer |
|---|---|
| Form Name | Fda 2579 Form |
| Form Length | 1 pages |
| Fillable? | No |
| Fillable fields | 0 |
| Avg. time to fill out | 15 sec |
| Other names | form fda 2579, 2579, fda form 2579, fda 2579 form pdf printable |
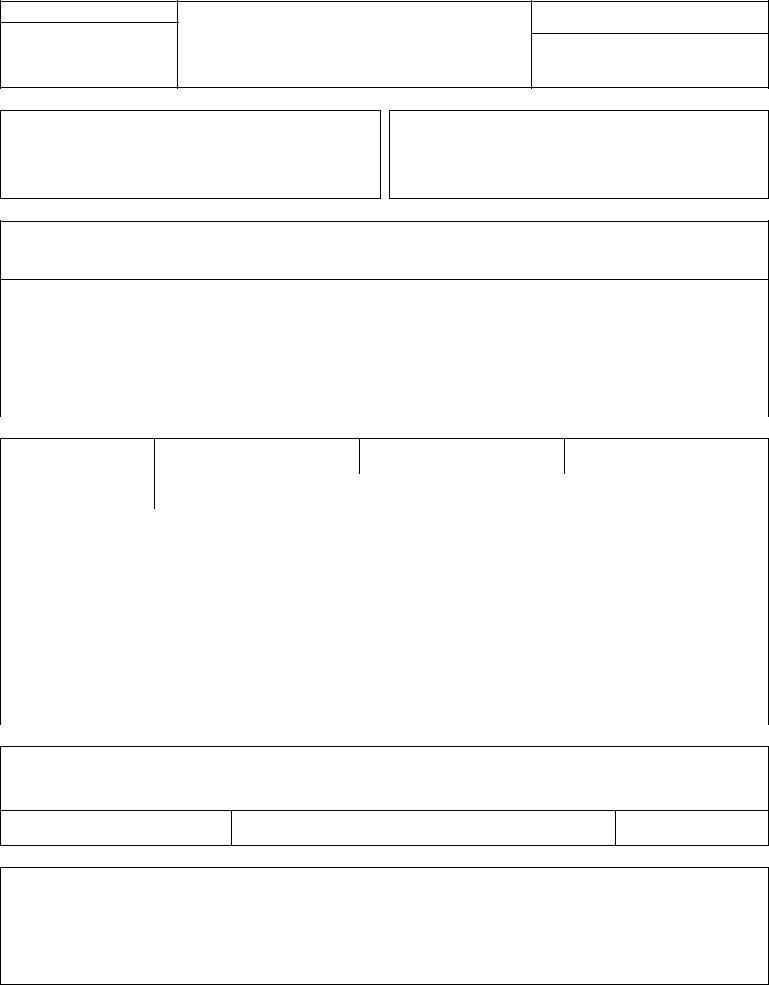
FOR FDA USE ONLY
DEPARTMENT OF HEALTH AND HUMAN SERVICES
Public Health Service
FOOD AND DRUG ADMINISTRATION
REPORT OF ASSEMBLY
OF A DIAGNOSTIC
Form Approved: OMB No.
Expiration Date: November 30, 2003
TEMPORARY
1. EQUIPMENT LOCATION
HOSPITAL, DOCTOR OR OFFICE WHERE INSTALLED
ABC Company
1111 First Street
Building 10
Rockville, 21704, US
Telephone:(111)
2. ASSEMBLER INFORMATION
COMPANY INFORMATION
DEF Company
2222 First Street
Building 20
Rockville, Province 9999999999, AR
Telephone:111 222 3333333333
3. GENERAL INFORMATION
THIS REPORT IS FOR ASSEMBLY OF CERTIFIED COMPONENTS WHICH ARE |
|
|
( ) |
(•) New |
( ) Replacement Components in an Existing System |
( ) |
( ) An Addition to an Existing System |
INTENDED USE(S) |
|
|
|
[X] General Purpose Radiology |
[X] Urology |
[X] CT Whole Body Scanner |
[X] Radiation Therapy Simulator |
[X] General Purpose Fluoroscopy |
[X] Mammography |
[X] |
[X] |
[X] Tomography (other than CT) |
[X] Chest |
[X] |
[X] Digital |
[X] Angiography |
[X] Chiropractic |
[X] |
[X] Bone Mineral Analysis |
[X] Podiatry |
[X] CT Headscanner |
[X] Dental Panoramic |
[X] |
[X] Other: Other Intended use.
THE X_RAY SYSTEM IS |
THE MASTER CONTROL IS IN ROOM |
DATE OF ASSEMBLY |
(•) Stationary ( ) Mobile |
Room A |
06/30/2008 |
|
|
|
4. COMPONENT INFORMATION
THE MASTER CONTROL IS |
CONTROL MANUFACTURER |
CONTROL SERIAL NUMBER |
DATE MANUFACTURED |
(•) A New Installation |
CM |
CSN |
|
|
|
|
06/2007 |
|
|
||
( ) Existing (Certified) |
|
|
|
|
|
|
|
|
|
||
CONTROL MODEL NUMBER |
|
|
SYSTEM MODEL NAME (CT Systems Only) |
|
|
||||||
( ) Existing |
|
|
|
|
|||||||
CMN |
|
|
CT SMN |
|
|
|
|||||
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
||||
|
|
|
SELECTED COMPONENTS |
|
|
|
OTHER CERTIFIED COMPONENTS (Number of each installed) |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MANUFACTURER |
|
MODEL NUMBER |
DATE MFR'ED |
[ 1] |
[ 6] |
Cradle |
|||
|
|
|
|
|
|
|
|||||
BEAM LIMITING |
|
MN B XXXXXXXXXX XXXXXXXXXX |
MDBXXXXXXXXXXXXXXXXX |
06/2007 |
[ 2] |
High Voltage |
[ 7] |
Film Changer |
|||
DEVICE |
XXXXXXXXXX XXXXXXXXXX XXXXXXXXXXX |
XXX |
|
|
|
|
Holder |
|
|
||
|
|
|
|
|
|
Generator |
|
|
|||
|
|
MN |
|
MD |
|
|
[ 3] |
Vertical Cassette |
[ 8] |
Image Intensifier |
|
|
|
MN |
|
MD |
|
|
[ 4] |
Tube Housing |
[ 9] |
Spot Film Device |
|
|
|
MN |
|
MD |
|
|
|||||
|
|
|
|
|
|
|
Assembly |
|
|
||
|
|
|
|
|
|
|
[ 5] |
Dental Tube Head |
[10] |
Fluoroscopic |
|
TABLES |
|
MANUFACTURER |
|
MODEL NUMBER |
DATE MFR'ED |
||||||
|
|
|
|
Device |
|
Imaging Assembly |
|||||
|
MN |
|
MD |
|
|
|
|
|
|||
|
|
MN T |
|
MD T |
06/2007 |
[11] |
Cephalometric |
[12] |
Image Receptor |
||
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
[13] |
Image Receptor |
[14] |
Fluorocopic Air |
CT GANTRY |
MANUFACTURER |
|
MODEL NUMBER |
DATE MFR'ED |
|
||||||
|
[15] |
Other: Other component |
Kerma Display |
||||||||
|
|
|
|
|
|
|
|
|
Support Device |
|
|
|
|
MN C |
|
MD C |
06/2007 |
|
|
|
|
Device |
|
|
|
|
|
|
|
|
|
|
|
|
|
5. ASSEMBLER CERTIFICATION
I affirm that all certified components assembled or installed by me, for which this report is being made, were adjusted and tested by me according to the instructions provided by the manufacture(s), were of the type required by the manufacture(s), were of the type required by the diagnostic
PRINTED NAME
John Smith
SIGNATURE
DATE
06/30/2008
6. COMMENTS
Comments....
Line 2
Line 3
Line 4
Line 5
Line 6
Line 7
Line 8
Line 9
Line 10