Using PDF files online is a breeze with our PDF tool. Anyone can fill in prior notice form here within minutes. To maintain our editor on the cutting edge of efficiency, we strive to put into action user-oriented features and improvements regularly. We're routinely thankful for any feedback - help us with revolutionizing PDF editing. Here is what you'll have to do to get going:
Step 1: Simply click the "Get Form Button" above on this page to launch our form editing tool. This way, you'll find all that is required to fill out your file.
Step 2: With our state-of-the-art PDF tool, you may do more than just fill out forms. Try all the functions and make your docs seem perfect with custom text added in, or modify the file's original content to excellence - all that comes along with an ability to add stunning pictures and sign it off.
This document will require particular data to be typed in, so ensure that you take the time to provide what's required:
1. To start with, when filling out the prior notice form, beging with the part that includes the next blank fields:
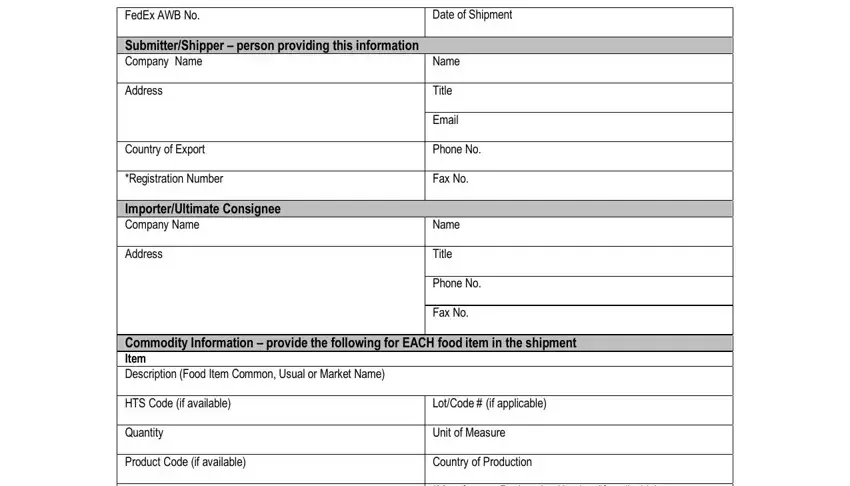
2. Just after this part is completed, go on to enter the relevant information in all these - Commodity Information cid provide, LotCode if applicable Unit of, Grower, Manufacturer, Name Address, Not required for shipments, and All food shipments regardless of.
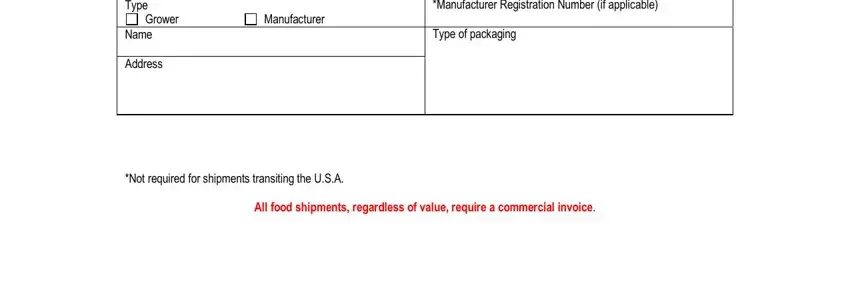
3. This stage is normally easy - complete all the blanks in FedEx AWB No Item Description Food, Grower, Manufacturer, Name Address Item Description Food, Date of Shipment, LotCode if applicable Unit of, and LotCode if applicable Unit of to conclude this part.
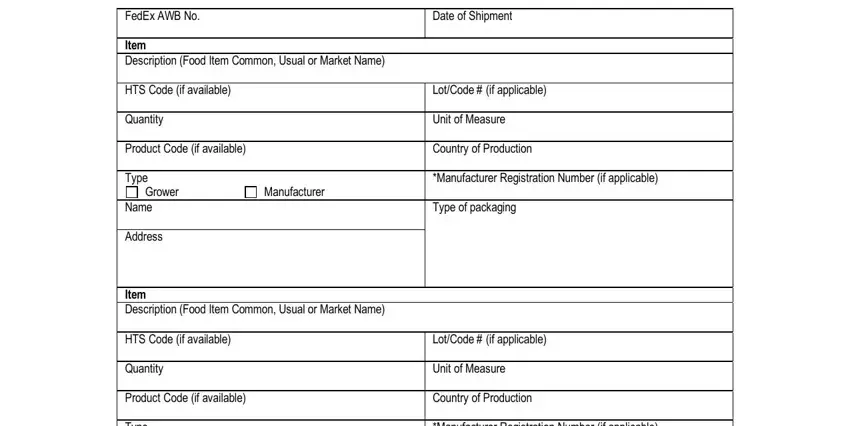
When it comes to FedEx AWB No Item Description Food and Date of Shipment, make sure you get them right in this current part. Both these are the most significant ones in this file.
4. This next section requires some additional information. Ensure you complete all the necessary fields - Name Address Item Description Food, Grower, Manufacturer, Name Address Item Description Food, Grower, Manufacturer, Name Address, LotCode if applicable Unit of, and LotCode if applicable Unit of - to proceed further in your process!
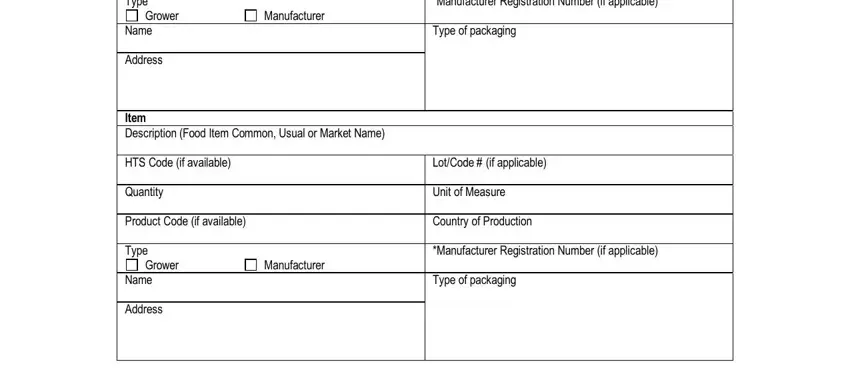
Step 3: Right after rereading your fields you have filled in, hit "Done" and you're good to go! After registering a7-day free trial account with us, you will be able to download prior notice form or send it via email immediately. The form will also be readily available through your personal account page with your every edit. When you work with FormsPal, you can certainly fill out forms without worrying about database breaches or entries being distributed. Our protected software helps to ensure that your personal details are kept safely.