INSTRUCTIONS
Effective July 1993, California law authorizes the California Department of Public Health (CDPH), Food and Drug Branch, to issue export documents upon request to California food, drug, medical device, and cosmetic firms wishing to export their products to other countries. Certificates are not required for export but are often required by the importing country. Documents are issued as follows:
Export Certificate or Certificate of Free Sale: Only for products manufactured in California facilities licensed, registered, permitted, or certified by the California Department of Public Health. The name of the manufacturer and the products will appear on the certificate.
Distributor Certificate: Only for products manufactured in California facilities licensed, registered, permitted, or certified by the California Department of Public Health. The name of the licensed, registered, permitted, or certified distributor and the products will appear on the certificate.
Certificate of Manufacture: Only for firms licensed, registered, permitted, or certified by the California Department of Public Health. No products or special wording are listed on this document. This document is not an export document but it may be used to demonstrate license, registration, permit, or certification status within CDPH.
You must complete the application form, provide appropriate information, sign the form, and pay the necessary fee(s) to obtain the export document. Instructions to complete this application are as follows:
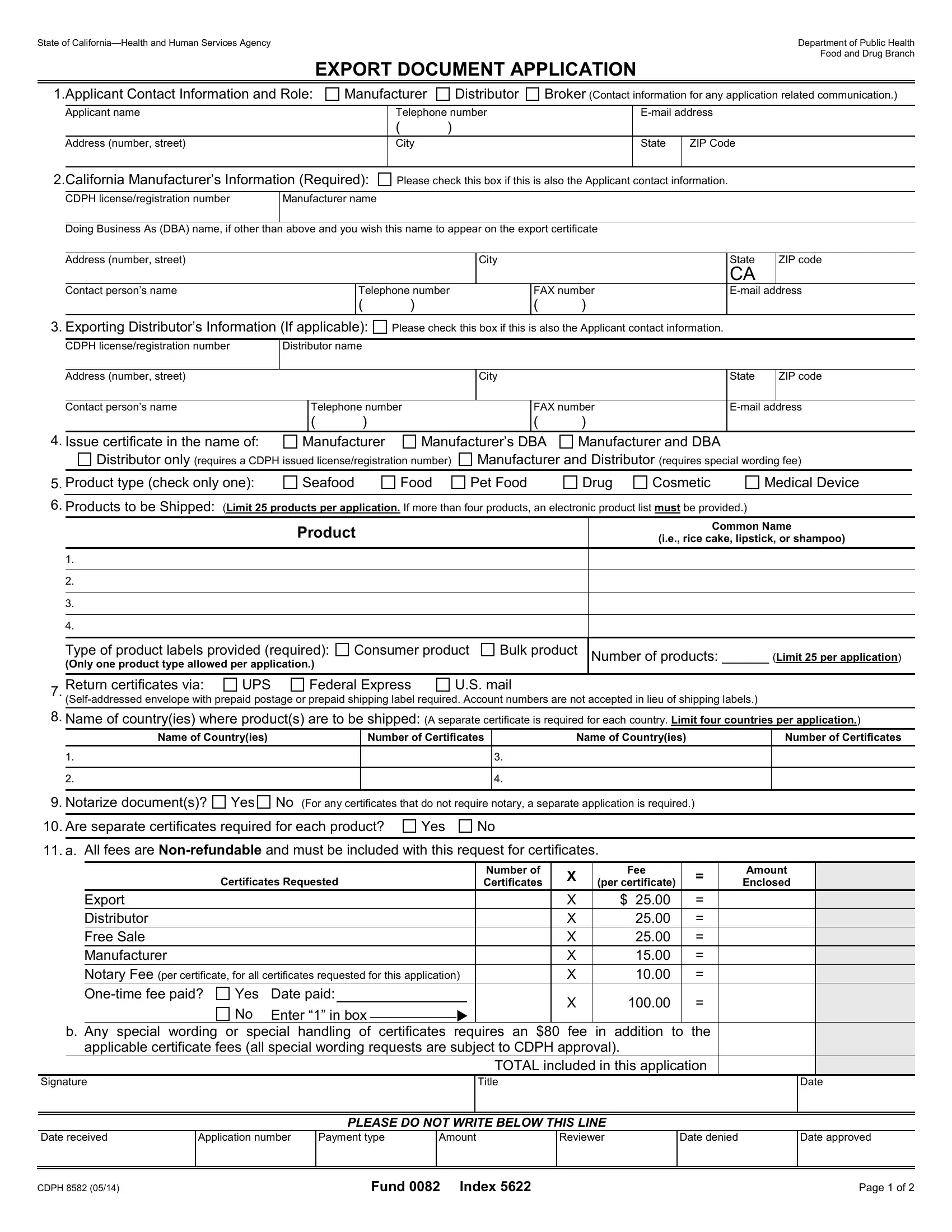
1.Applicant Contact Information and Role. This information will be used as the contact information for any and all correspondence regarding the application.
2.California Manufacturer’s Information CDPH issued license/registration number.
Manufacturer name: This is the exact name of the product manufacturer whose name appears on a license, registration, permit, or certification issued by the California Department of Public Health. If desired, this name will appear on the export documents.
Doing Business As (DBA): If you would like to have a doing business as (DBA) name on the export certificate, please indicate the name you wish to appear.
Address: Address of the product manufacturer whose name appears on a license, registration, permit, or certification issued by the California Department of Public Health.
Contact person’s name, telephone number, FAX number, and e-mail address.
3.Exporting Distributor’s Information: (if applicable)
CDPH issued license/registration number. PLEASE NOTE: If it is requested that the distributor’s information only should be listed on the certificate, then the distributor must have a CDPH issued license number OR an $80 special handling fee must be paid and the licensed/registered manufacturer AND unlicensed/unregistered distributor names will both be listed on the export, distributor, or free sale certificates.
Distributor name: This is the name of the party distributing the product. Please provide evidence that the particular lot of the product(s) was manufactured by the product manufacturer (e.g., a copy of invoice from the manufacturer).
Address: Address of the party distributing the product. Distributor’s Contact name and telephone number.
4.Issue certificate in the name of: Choose from the options listed. PLEASE NOTE: If it is requested that the distributor’s information only should be listed on the certificate, then the distributor must have a CDPH issued license number OR an $80 special handling fee must be paid and the licensed manufacturer AND unlicensed distributor names will both be listed.
5.Product type: Select only one product type. A separate application is required for each product type.
6.Products to be shipped: State the product name that exactly matches the name on the product label. This name will appear on any export, free sale, or distributor
certificates. Also state the common or usual name of the product(s). PLEASE NOTE: If the application has more than 4 products, please submit an electronic product list on either a CD, thumb drive, or via email to FDBExports@cdph.ca.gov along with the application. We will only accept electronic copies in either word or excel formats.
Product Labels: All labels, labeling, and advertising affixed to, accompanying, or relating to the food, drug, device, or cosmetic must be attached to the application for each product. The department shall accept electronic or paper copies of labels, labeling or advertising. Please submit labels for only one product type (e.g.,
Consumer product or Bulk product) per application.
Number of Products: Total number of products you are listing on the application. Limit of 25 products per application.
7.Return Certificates Via: Please indicate the mode of return shipping for the certificates requested. A self-addressed envelope with prepaid postage or a prepaid shipping label is required to be included with the application for return shipping.
8.Name of country(ies) where product(s) are to be shipped: (A separate certificate is required for each country. Limit of four countries per application.)
9.Notarize document(s)? Notary Fees are $10 per certificate. If document(s) is/are to be notarized, include notary fees for each certificate. Any documents that do not require notarization must be submitted on a separate application.
10.Are separate certificates requested for each product? If you would like a separate certificate for each product, please check “yes.”
11.All fees are Non-refundable. Please submit one check per application. Please calculate your fees based on the following: Export, Free Sale, and Distributor certificates: $25 per certificate.
Manufacturer certificates: $15 per certificate.
Notary fees: $10 per certificate. Applies to ALL certificates requested for each application. Any certificates that do not require notary will need to be included in a separate application.
One-time Fee: One one-time fee of $100.00 is required for the first application submitted for each CDPH license/registration number. If this fee has already been paid, you must indicate the date paid.
Special wording: An $80 special wording fee is required for any special wording requests or special handling of the requested documents. All special wording is subject
to CDPH approval.
Please sign, date, and print title of signatory. Mail or ship the application with product labels, product list, shipping label and the appropriate fees to:
Via mail: |
California Department of Public Health |
Via Fed Ex, UPS, etc. California Department of Public Health |
|
Food and Drug Branch – Export Document Program |
Food and Drug Branch |
|
MS 7602 |
Export Document Program |
|
P.O. Box 997435 |
1500 Capitol Avenue, MS 7602 |
|
Sacramento, CA 95899-7435 |
Sacramento, CA 95814 |
If you have any questions, please contact the Export Document Program, Food and Drug Branch at FDBExports@cdph.ca.gov or (916) 650-6519. Export Document Application requirements and Frequently Asked Questions may be found at http://www.cdph.ca.gov/programs/Pages/FDBExportDocumentProgram.aspx.
CDPH 8582 (05/14) |
Fund 0082 Index 5622 |
Page 2 of 2 |