In the sphere of occupational health and safety within the nuclear industry, the BRC Form 202-3 plays a pivotal role, ensuring the thorough documentation and monitoring of radiation exposure among workers. Crafted by the Texas Department of Health/Bureau of Radiation Control in October 2000, this form is meticulously designed to record detailed information on occupational exposure during a specific monitoring period. It encompasses a wide array of data, including personal identification details of the monitored individual, the nature of their exposure, and precise dosage information across various categories, such as deep dose equivalent (DDE) and committed effective dose equivalent (CEDE). Furthermore, it allows for distinguishing between routine monitoring and special exposure events, ensuring that both ongoing exposure levels and specific incident-related doses are accurately captured. Whether for regulatory compliance, health monitoring, or ensuring workplace safety, the BRC Form 202-3 serves as an essential instrument for managing and mitigating radiation exposure risks, providing a critical link between occupational health practices and radiological safety protocols.
| Question | Answer |
|---|---|
| Form Name | Brc Form 202 3 |
| Form Length | 2 pages |
| Fillable? | No |
| Fillable fields | 0 |
| Avg. time to fill out | 30 sec |
| Other names |
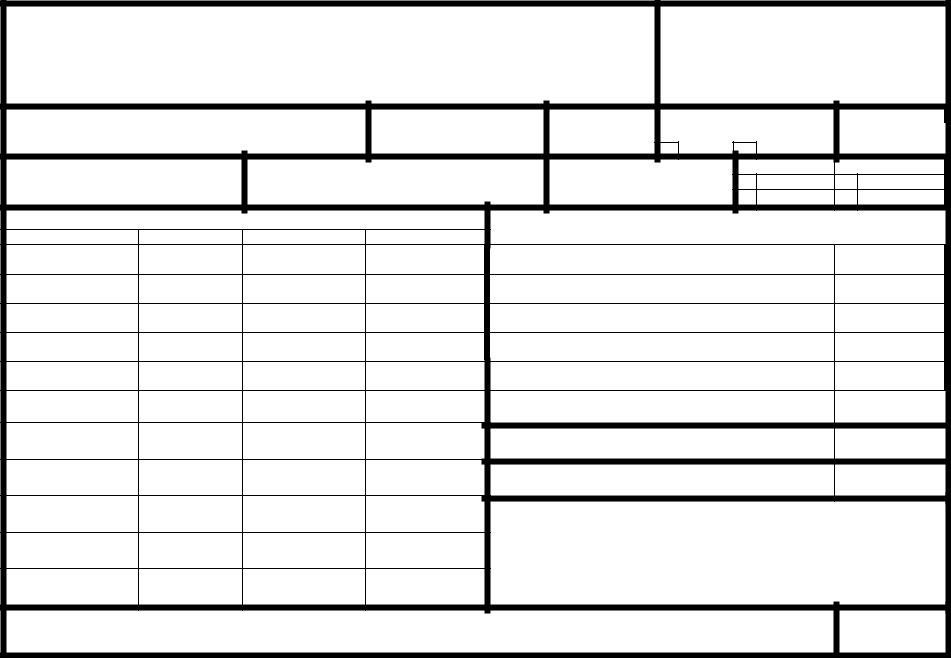
PAGE ______ OF ______
BRC Form |
|
|
Texas Department of Health/Bureau of Radiation Control |
|
|
|
|
|
(October 2000) |
|
|
|
|
|
|
|
|
|
OCCUPATIONAL EXPOSURE RECORD |
|
|
|
|
|
||
|
FOR A MONITORING PERIOD |
|
|
|
|
|
||
1. NAME (LAST, FIRST, MIDDLE INITIAL) |
|
2. IDENTIFICATION NUMBER |
3. ID TYPE |
4. SEX |
|
|
5. DATE OF BIRTH |
|
|
|
|
|
|
MALE |
FEMALE |
|
|
6. MONITORING PERIOD |
|
7. LICENSEE OR REGISTRANT NAME |
8. LICENSE OR REGISTRATION |
9A. |
|
9B. |
||
|
|
|
|
NUMBER(S) |
|
RECORD |
ROUTINE |
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
ESTIMATE |
PSE |
|
|
INTAKES |
|
|
DOSES (IN REM) |
|
|
||
|
|
|
|
|
|
|
||
10A. RADIONUCLIDE |
10B. CLASS |
10C. MODE |
10D. INTAKE IN FCi |
|
|
|
|
|
|
|
|
DEEP DOSE EQUIVALENT |
|
|
(DDE) |
11. |
|
|
|
|
|
|
|
|||
|
|
|
EYE DOSE EQUIVALENT TO THE LENS OF THE EYE |
|
(LDE) |
12. |
||
|
|
|
|
|
||||
|
|
|
SHALLOW DOSE EQUIVALENT, WHOLE BODY |
(SDE,WB) |
13. |
|||
|
|
|
|
|||||
|
|
|
SHALLOW DOSE EQUIVALENT, MAX EXTREMITY |
(SDE,ME) |
14. |
|||
|
|
|
|
|||||
|
|
|
COMMITTED EFFECTIVE DOSE EQUIVALENT |
|
(CEDE) |
15. |
||
|
|
|
|
|
||||
|
|
|
COMMITTED DOSE EQUIVALENT, |
|
|
16. |
||
|
|
|
MAXIMALLY EXPOSED ORGAN |
|
|
(CDE) |
|
|
|
|
|
TOTAL EFFECTIVE DOSE EQUIVALENT |
|
|
17. |
||
|
|
|
|
|
(BLOCKS 11+15) |
(TEDE) |
|
|
|
|
|
TOTAL ORGAN DOSE EQUIVALENT, |
|
|
18. |
||
|
|
|
MAX ORGAN |
(BLOCKS 11+16) |
(TODE) |
|
||
|
|
|
19. COMMENTS |
|
|
|
|
|
20. SIGNATURE |
|
|
|
|
|
|
21. DATE PREPARED |
|
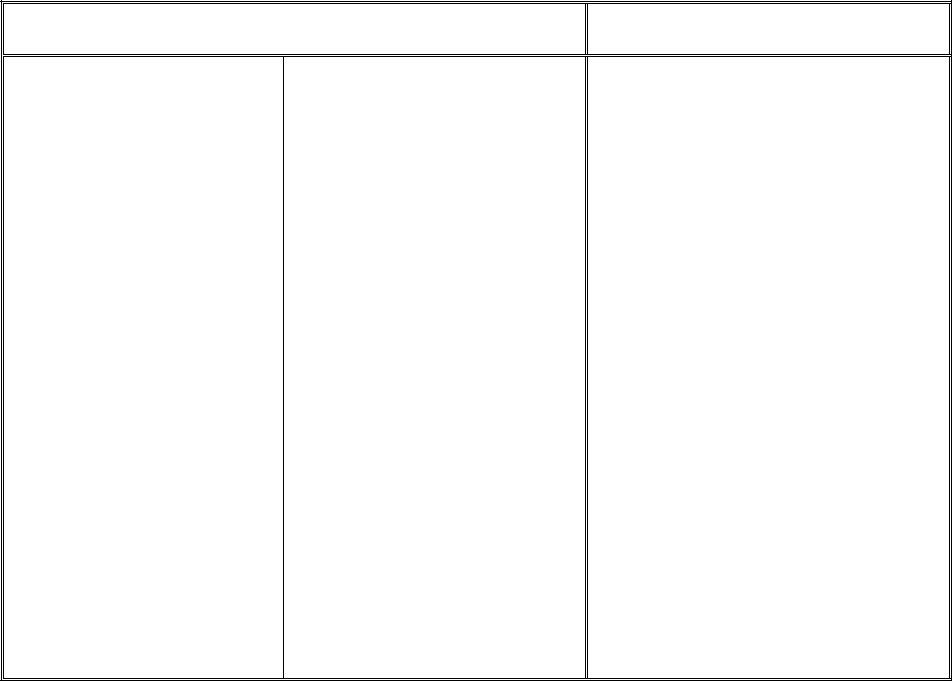
INSTRUCTIONS AND ADDITIONAL INFORMATION PERTINENT TO THE
COMPLETION OF BRC FORM
(ALL DOSES SHOULD BE STATED IN REMS)
1.Type or print the full name of the monitored individual in the order of last name (include "Jr," "Sr," "III," etc.), first name, middle initial (if applicable).
2.Enter the individual's identification number, including punctuation. This number should be the
3.Enter the code for the type of identification used as shown below:
CODE |
ID TYPE |
|
SSN |
U.S. Social Security Number |
|
PPN |
Passport Number |
|
CSI |
Canadian Social Insurance Number |
|
WPN |
Work Permit Number |
|
IND |
INDEX Identification Number |
|
OTH |
Other |
|
4.Check the box that denotes the sex of the individual being monitored.
5.Enter the date of birth of the individual being monitored in the format MM/DD/YY.
6.Enter the monitoring period for which this report is filed. The format should be MM/DD/YY - MM/DD/YY.
7.Enter the name of the licensee or registrant.
8.Enter the Agency license or registration number or numbers.
9A. Place an "X" in Record or Estimate. Choose "Record" if the dose data listed represent a final determination of the dose received to the best of the licensee's or registrant's knowledge. Choose "Estimate" only if the listed dose data are preliminary and will be superseded by a final determination resulting in a subsequent report. An example of such an instance would be dose data based on
9B. Place an "X" in either Routine or PSE. Choose "Routine" if the data represent the results of monitoring for routine exposures. Choose "PSE" if the listed dose data represents the results of monitoring of planned special exposures received during the monitoring
period. If more than one PSE was received in a single year, the licensee or registrant should sum them and report the total of all PSEs.
10A. Enter the symbol for each radionuclide that resulted in an internal exposure recorded for the individual, using the format
10B. Enter the lung clearance class as listed in Appendix B to Part D (D, W, Y, V, or O for other) for all intakes by inhalation.
10C. Enter the mode of intake. For inhalation, enter "H." For absorption through the skin, enter "B." For oral ingestion, enter "G." For injection, enter "J."
10D. Enter the intake of each radionuclide in FCi.
11.Enter the deep dose equivalent (DDE) to the whole body.
12.Enter the eye dose equivalent (LDE) recorded for the lens of the eye.
13.Enter the shallow dose equivalent recorded for the skin of the whole body (SDE,WB).
14.Enter the shallow dose equivalent recorded for the skin of the extremity receiving the maximum dose (SDE,ME).
15.Enter the committed effective dose equivalent (CEDE) or "NR" for "Not Required" or "NC" for "Not Calculated".
16.Enter the committed dose equivalent (CDE) recorded for the maximally exposed organ or "NR" for "Not Required" or "NC" for "Not Calculated".
17.Enter the total effective dose equivalent (TEDE). The TEDE is the sum of items 11 and 15.
18.Enter the total organ dose equivalent (TODE) for the maximally exposed organ. The TODE is the sum of items 11 and 16.
19.COMMENTS.
In the space provided, enter additional information that might be needed to determine compliance with limits. An example might be to enter the note that the SDE,ME was the result of exposure from a discrete hot particle. Another possibility would be to indicate that an overexposed report has been sent to the Agency in reference to the exposure report.
20.Signature of the person designated to represent the licensee or registrant.
21.Enter the date this form was prepared.