The Clinical Experience form plays a critical role in the certification process for individuals aspiring to excel in the field of Computed Tomography (CT). Beginning July 1, 2011, with subsequent edits in July 2012 and June 2013, the purpose behind these requirements is to ensure candidates possess hands-on experience in performing a range of specific clinical procedures crucial to their discipline. This hands-on experience, coupled with the mastery of cognitive knowledge and skills assessed by the certification examination, lays the foundation for acquiring a comprehensive skill set applicable across various settings. The documentation form, or a reasonable facsimile approved by the American Registry of Radiologic Technologists® (ARRT®), is used to record the execution of these procedures within a 24-month period prior to application submission—highlighting the importance of practical experience in the certification journey. The form meticulously records each procedure’s details, ensuring that candidates not only perform but also document their clinical experiences accurately, which are then verified by a qualified professional. It's clear that the pathway to certification demands not just theoretical knowledge but a significant emphasis on verified clinical practice, which ultimately contributes to the competency and proficiency of future radiologic technologists.
| Question | Answer |
|---|---|
| Form Name | Clinical Experience Form |
| Form Length | 7 pages |
| Fillable? | No |
| Fillable fields | 0 |
| Avg. time to fill out | 1 min 45 sec |
| Other names | ct clinical experience document, ct clinical experience documentation form, Technologist, colonography |

COMPUTED TOMOGRAPHY
CLINICAL EXPERIENCE REQUIREMENTS
Eligibility Requirements Effective
for Examinations Beginning July 1, 2011*
Edited July 2012
Edited June 2013
The purpose of the clinical experience requirements is to verify that candidates have completed a subset of the clinical procedures within a discipline. Successful performance of these fundamental procedures, in combination with mastery of the cognitive knowledge and skills covered by the certification examination, provides the basis for the acquisition of the full range of clinical skills required in a variety of settings.
This document identifies the clinical experience required for certification. The American Registry of Radiologic Technologists® (ARRT®) encourages individuals to obtain education and experience beyond these minimum requirements.
Instructions for Eligibility
1.Perform the Procedures: Candidates are required to perform clinical procedures according to the Specific Procedural Requirements on the following pages. All procedures must be performed within the 24 month period immediately preceding the date that the application is received by ARRT. Repetitions may be completed in less than 24 months.
2.Document Performance: Candidates must use the following Computed Tomography Clinical Experience Documentation Form or a reasonable facsimile to record the performance of the procedures. Documentation includes: name of procedure, date performed, time of day completed, facility where performed, and the initials of the person verifying performance. The “Verifier’s Initials” column on the form must be initialed by a Registered Technologist, supervisor, or a licensed physician and must match the Verification Identification Page at the end of this document. The name and address of the person corresponding to each set of initials must also be provided on the Verification Identification Page. Failure to meet the minimum clinical experience requirements prior to the date of submission will result in the application becoming “ineligible.” All documented procedures prior to the date the application is received by ARRT will not be accepted for future clinical experience requirements.
3.Apply for Certification: After the clinical experience requirements have been completed, candidates are eligible to complete the application for certification. Submitting false documentation to ARRT as part of the application process is a violation of the ARRT Standards of Ethics and may result in sanctions up to and including revocation of ARRT certification in all disciplines and ineligibility for any additional ARRT certifications.
4.Maintain Your Records: Candidates must keep the Clinical Experience Documentation Form for at least 24 months after the date the application is submitted. The ARRT conducts audits of some applications for certification. Candidates who are audited will be required to send the Clinical Experience Documentation Form to ARRT. Additional documentation may be required from individuals who are audited.
*Note: Candidates who submit their applications up through June 30, 2013 may use either the previous requirements (effective July 2008) or the current requirements (effective 2011). Candidates who apply after June 30, 2013 may no longer use the previous clinical experience requirements.
Copyright © 2012 by The American Registry of Radiologic Technologists. All rights reserved.
Specific Procedural Requirements
The Clinical Experience Requirements for CT consist of 59 procedures in 7 different categories:
A.Head and Neck
B.Spine and Musculoskeletal
C.Chest
D.Abdomen and Pelvis
E.Special Procedures
F.Image Display and Post Processing
G.Quality Assurance
Candidates must document the performance of complete, diagnostic quality procedures according to the following rules:
Choose a minimum of 25 different procedures out of the 59 procedures on the following pages.
Complete and document a minimum of 3 and a maximum of 5 repetitions of each chosen procedure; less than three will not be counted toward the total.
No more than one procedure may be documented on one patient. For example, if an order requests chest, abdomen, and pelvis scans for one patient, only one of these may be documented for clinical experience documentation.
Computed Tomography procedures performed in conjunction with a PET or SPECT procedure or Radiation Therapy planning procedure must be of diagnostic quality.
A minimum total of 125 repetitions across all procedures is required.
Examples
1)A candidate who works in a specialized setting wanted to complete the minimum number of procedures. This person chose 25 procedures from any of the 7 categories. To complete 125 repetitions, each of the 25 procedures was performed 5 times each.
2)Another candidate works in a facility that does most types of CT scans, so completing a wide variety of procedures was quite feasible. This candidate completed a total of 30 procedures from all 7 categories. Although most of these procedures were performed 3 times (the minimum), several of them were performed 4 or 5 times each until the candidate reached at least 125 procedures.
General Guidelines
To qualify as a complete, diagnostic quality CT imaging procedure the candidate must demonstrate appropriate:
evaluation of requisition and/or medical record preparation of examination room identification of patient
patient assessment and education concerning the procedure documentation of patient history including allergies patient positioning
protocol selection parameter selection
image display, filming, and archiving
documentation of procedure, treatment and patient data in appropriate record patient discharge with
standard precautions radiation safety
preparation and/or administration of contrast media initiate scan
and evaluate the resulting images for:
image quality (e.g., motion, artifacts, noise)
optimal demonstration of anatomic region (e.g., delayed imaging, reconstruction spacing, algorithm, slice thickness)
exam completeness
Attention CT Certification Candidates: Your certification process has requirements to complete clinical procedures including activating actual CT scans, known as “initiating the scan” or “making the exposure.” You are responsible for ensuring state laws allow you to complete this requirement.
Computed Tomography
Clinical Experience Requirement Procedures
A.Head and Neck
1.head without and/or with contrast
2.sinuses
3.facials (orbits, mandible)
4.temporal bones / IACs
5.trauma head
6.vascular head (CTA)
7.soft tissue neck
8.vascular neck (CTA)
B.Spine and Musculoskeletal
1.lumbar
2.cervical
3.thoracic
4.spinal trauma
5.upper extremity
6.lower extremity
7.pelvic girdle; hips
8.musculoskeletal trauma
9.vascular extremity (CTA)
C.Chest
1.chest without and/or with contrast
2.HRCT
3.vascular chest (e.g., PE, CTA, Aorta)
4.chest trauma
5.lung nodule study
6.heart (e.g., calcium scoring, coronary angiography)
D.Abdomen and Pelvis
1.abdomen without and/or with contrast
2.liver
3.kidneys
4.pancreas
5.adrenals
6.enterography study
7.appendicitis study
8.renal stone protocol (without IV contrast)
9.abdominal trauma
10.vascular abdomen (CTA)
11.CT intravenous urogram/IVP
12.pelvis without and/or with contrast
13.bladder
14.pelvic trauma
15.vascular pelvis (CTA)
16.colorectal studies (rectal contrast)
E.Special Procedures
1.biopsies
2.drainage
3.aspirations
4.CT arthrography
5.diskography
6.myelography
7.colonography or virtual colonography
8.brain perfusion
9.radiation therapy planning
10.transplant studies
F.Image Display and Post Processing
1.geometric or distance measurements
2.region of interest measurement (ROI)
3.retrospective reconstruction
4.multiplanar reconstruction (MPR)
5.3D rendering (MIP, SSD, VR)
G.Quality Assurance
1.calibration checks
2.CT number and standard deviation (water phantom)
3.linearity
4.spatial resolution
5.contrast resolution
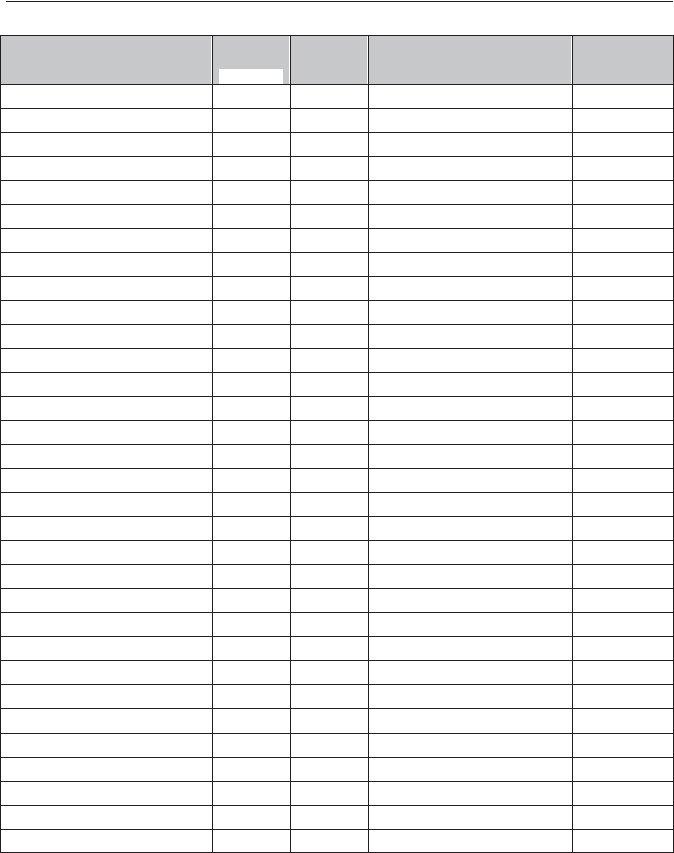
Clinical Experience Documentation Form
Computed Tomography
CANDIDATE NAME |
ARRT ID # |
All procedures must be performed on patients (not phantoms or simulated patients). Procedures must be verified and initialed by a Registered Technologist, supervisor, or a licensed physician and must match the Verification Identification Page at the end of this document. The name and address of the person corresponding to each set of initials must also be provided on the Verification Identification Page. List procedures in the order they are listed on the preceding page, with like procedures grouped together. See the example below. If all of your clinicals are completed at the same facility, documenting the facility name once is sufficient. Only those procedures completed within the 24 months preceding the date the application is received by ARRT will be accepted.
|
|
|
|
|
|
|
|
Verifier’s |
Category and Procedure |
|
Date |
|
Time of |
|
|
Initials |
|
Performed |
|
mm/dd/yy |
|
Day |
Facility Name |
|
(handwritten) |
|
HEAD: head without contrast |
|
09/01/12 |
|
|
10:15 a.m. |
General Hospital |
|
|
|
|
|
|
|
|
|
|
|
head without and with |
|
|
|
3:00 p.m. |
|
|
|
|
|
09/01/12 |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
head without contrast |
|
09/02/12 |
|
|
8:00 a.m. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
This form may be duplicated
CANDIDATE NAME |
ARRT ID # |
|
|
|
|
Verifier’s |
Category and Procedure |
Date |
Time of |
|
Initials |
Performed |
mm/dd/yy |
Day |
Facility Name |
(handwritten) |
This form may be duplicated
CANDIDATE NAME |
ARRT ID # |
VERIFICATION IDENTIFICATION PAGE
The previous pages of the Computed Tomography Clinical Experience Documentation Form require only that the initials of the person verifying performance of a procedure be listed. On this page, the verifiers must provide their full name and mailing address to match their initials on the previous pages. These individuals may be contacted as part of the audit process. Registered Technologists should list their home mailing address that is on file with ARRT. Other verifiers may list the facility address.
Verifier’s Initials |
Verifier’s Initials |
(handwritten) |
(handwritten) |
|
|
Verifying technologist ARRT ID # and credentials (if applicable) |
Verifying technologist ARRT ID # and credentials (if applicable) |
Others, please note credentials in this space |
Others, please note credentials in this space |
|
|
Printed Name |
Printed Name |
|
|
Mailing Address |
Mailing Address |
|
|
City/State/Zip |
City/State/Zip |
|
|
Verifier’s Initials |
Verifier’s Initials |
(handwritten) |
(handwritten) |
|
|
Verifying technologist ARRT ID # and credentials (if applicable) |
Verifying technologist ARRT ID # and credentials (if applicable) |
Others, please note credentials in this space |
Others, please note credentials in this space |
|
|
Printed Name |
Printed Name |
|
|
Mailing Address |
Mailing Address |
|
|
City/State/Zip |
City/State/Zip |
|
|
Verifier’s Initials |
Verifier’s Initials |
(handwritten) |
(handwritten) |
|
|
Verifying technologist ARRT ID # and credentials (if applicable) |
Verifying technologist ARRT ID # and credentials (if applicable) |
Others, please note credentials in this space |
Others, please note credentials in this space |
|
|
Printed Name |
Printed Name |
|
|
Mailing Address |
Mailing Address |
|
|
City/State/Zip |
City/State/Zip |
|
|
Verifier’s Initials |
Verifier’s Initials |
(handwritten) |
(handwritten) |
|
|
Verifying technologist ARRT ID # and credentials (if applicable) |
Verifying technologist ARRT ID # and credentials (if applicable) |
Others, please note credentials in this space |
Others, please note credentials in this space |
|
|
Printed Name |
Printed Name |
|
|
Mailing Address |
Mailing Address |
|
|
City/State/Zip |
City/State/Zip |
|
|
This form may be duplicated