In the realm of ensuring food safety and quality within the United States Army, the DA Form 7539 plays a pivotal role, tasked as the "Request for Veterinary Laboratory Testing & Food Sample Record." Governed by the procedural directive AR 40-657, with the Office of The Surgeon General (OTSG) as the proponent agency, this form serves as a crucial link between food providers and the rigorous safety standards enforced by military regulations. Its fields capture a comprehensive range of information, starting from basic identification details of the submitting unit and point of contact, extending through to the intricate specifics of the food sample itself, including the producer or manufacturer details, reasons for submission—such as suspected foodborne illness, sanitation audits, or contract compliance—through to the conditions under which the samples were shipped to the laboratory. The specific laboratories designated to receive these submissions are detailed, including locations in Bahrain, Hawaii, Korea, among others, ensuring a global reach in monitoring food safety. Furthermore, the form provides space for in-depth details regarding the sample, like product codes, brand names, and sample weight/volume, allowing for a meticulous analysis by the receiving laboratory. Rightfully, the DA 7539 form encapsulates a crucial process in upholding food quality and safety standards within army operations, embodying the interdisciplinary cooperation between logistical, health, and safety protocols.
| Question | Answer |
|---|---|
| Form Name | Da Form 7539 |
| Form Length | 2 pages |
| Fillable? | No |
| Fillable fields | 0 |
| Avg. time to fill out | 30 sec |
| Other names | SUBMITTER, OTSG, IMSL, 9V1 |
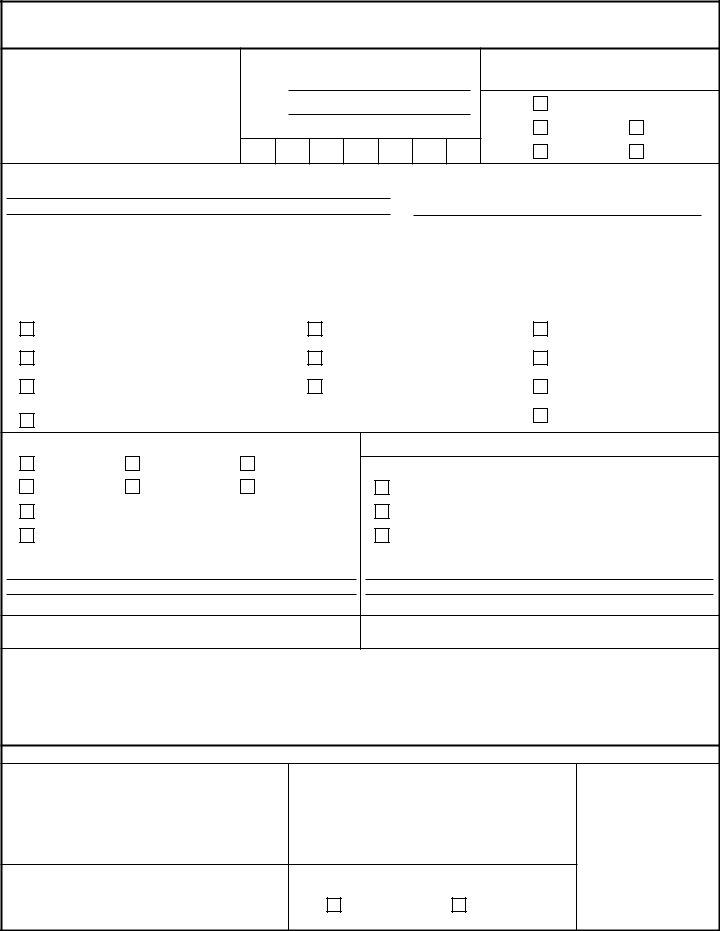
REQUEST FOR VETERINARY LABORATORY TESTING & FOOD SAMPLE RECORD
For use of this form, see AR
1 . FROM:
2 . POINT OF CONTACT:
Name:
Phone:
Station Identification Number:
-
3 . CONTROL NUMBER:
4 . TO: |
VETCOM FADL |
|
|
VLE |
BAHRAIN |
|
HAWAII |
KOREA |
5 . PRODUCER/MANUFACTURER (Name, Address and Phone):
|
|
|
|
|
ESTABLISHMENT # / PLANT CODE (IMSL, USDA, etc.) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
VC # |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
6 . REASON FOR SUBMISSION: |
|
|
|
Sanitation Audits |
|
||
|
|
|
|
|
|||
|
Suspected foodborne illness |
Destination monitoring program |
Initial |
|
|||
|
(contact laboratory prior to submission) |
|
|
|
|
|
|
|
Suspected foreign material/object |
Contract compliance |
Special |
|
|||
|
Customer return/complaint |
Proximate analysis |
Directed routine |
|
|||
|
(provide synopsis of incident/problem and local |
|
|
|
|
|
|
|
inspection results in the Remarks section below ). |
|
|
|
Routine |
|
|
|
OTHER (Specif y): |
|
|
|
|
||
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
7 . SAMPLES SELECTED FROM:
DECA |
MWR |
Exchange |
Exchange vendor |
Commercial establishment
OTHER:
PLANT
Prime vendor
8 . DATE SAMPLE(S) SELECTED: |
THRU |
9 . SHIPMENT TEMPERATURE CONDITIONS:
Room temperature
Frozen
Chilled - include 1 temperature pilot per shipping container
10 . INSPECTOR' S SIGNATURE
11 . ACCOUNTABLE OFFICER' S SIGNATURE
12 . REMARKS (use additional paper if necessary):
FOR LABORATORY USE ONLY
SHIPPING CARRIER TRACKING NUMBER: |
LABORATORY REPORT NUMBER: |
SAMPLE(S) FOR ANALYSIS BY:
RECEIPT TEMPERATURE: |
|
CHEMISTRY |
MICROBIOLOGY |
|
|
|
|
RECEIVED:
DA FORM 7539, FEB 2005
APD 9V1.000
PAGE 1 OF 2

13 . SAMPLE INFORMATION (Complete as much information as is available): |
LAB REPORT # |
|
||
|
|
|
|
|
SAMPLE NUMBER 1 |
|
FOR LABORATORY USE ONLY |
|
|
|
|
|
|
|
|
|
|
|
|
SUBMITTER SAMPLE NUMBER |
SAMPLE DESCRIPTION |
|
|
BRAND NAME |
|
|
|
|
|
UNIVERSAL PRODUCT CODE (UPC) |
PRODUCT CODE |
|
|
SAMPLE WEIGHT/VOLUME |
|
|
|
|
|
QUANTITY SUBMITTED |
UNIT OF ISSUE |
TOTAL COST |
DISPOSITION |
|
|
|
|
|
|
SAMPLE NUMBER 2 |
|
FOR LABORATORY USE ONLY |
|
|
|
|
|
|
|
|
|
|
|
|
SUBMITTER SAMPLE NUMBER |
SAMPLE DESCRIPTION |
|
|
BRAND NAME |
|
|
|
|
|
UNIVERSAL PRODUCT CODE (UPC) |
PRODUCT CODE |
|
|
SAMPLE WEIGHT/VOLUME |
|
|
|
|
|
QUANTITY SUBMITTED |
UNIT OF ISSUE |
TOTAL COST |
DISPOSITION |
|
|
|
|
|
|
SAMPLE NUMBER 3 |
|
FOR LABORATORY USE ONLY |
|
|
|
|
|
|
|
|
|
|
|
|
SUBMITTER SAMPLE NUMBER |
SAMPLE DESCRIPTION |
|
|
BRAND NAME |
|
|
|
|
|
UNIVERSAL PRODUCT CODE (UPC) |
PRODUCT CODE |
|
|
SAMPLE WEIGHT/VOLUME |
|
|
|
|
|
QUANTITY SUBMITTED |
UNIT OF ISSUE |
TOTAL COST |
DISPOSITION |
|
|
|
|
|
|
SAMPLE NUMBER 4 |
|
FOR LABORATORY USE ONLY |
|
|
|
|
|
|
|
|
|
|
|
|
SUBMITTER SAMPLE NUMBER |
SAMPLE DESCRIPTION |
|
|
BRAND NAME |
|
|
|
|
|
UNIVERSAL PRODUCT CODE (UPC) |
PRODUCT CODE |
|
|
SAMPLE WEIGHT/VOLUME |
|
|
|
|
|
QUANTITY SUBMITTED |
UNIT OF ISSUE |
TOTAL COST |
DISPOSITION |
|
|
|
|
|
|
SAMPLE NUMBER 5 |
|
FOR LABORATORY USE ONLY |
|
|
|
|
|
|
|
|
|
|
|
|
SUBMITTER SAMPLE NUMBER |
SAMPLE DESCRIPTION |
|
|
BRAND NAME |
|
|
|
|
|
UNIVERSAL PRODUCT CODE (UPC) |
PRODUCT CODE |
|
|
SAMPLE WEIGHT/VOLUME |
|
|
|
|
|
QUANTITY SUBMITTED |
UNIT OF ISSUE |
TOTAL COST |
DISPOSITION |
|
|
|
|
|
|
SAMPLE NUMBER 6 |
|
FOR LABORATORY USE ONLY |
|
|
|
|
|
|
|
|
|
|
|
|
SUBMITTER SAMPLE NUMBER |
SAMPLE DESCRIPTION |
|
|
BRAND NAME |
|
|
|
|
|
UNIVERSAL PRODUCT CODE (UPC) |
PRODUCT CODE |
|
|
SAMPLE WEIGHT/VOLUME |
|
|
|
|
|
QUANTITY SUBMITTED |
UNIT OF ISSUE |
TOTAL COST |
DISPOSITION |
|
|
|
|
|
|
|
FOR ADDITIONAL SAMPLES, USE ADDITIONAL COPIES OF PAGE 2. |
DA FORM 7539, FEB 2005 |
APD 9V1.000 |
|
PAGE 2 OF 2 |