Working with PDF documents online is certainly easy with our PDF tool. Anyone can fill in Dpr Form 39 030 here and try out various other functions available. To keep our tool on the forefront of convenience, we aim to adopt user-driven capabilities and enhancements regularly. We're routinely grateful for any feedback - join us in remolding how we work with PDF docs. All it requires is several simple steps:
Step 1: Simply click on the "Get Form Button" at the top of this page to open our pdf form editor. Here you'll find all that is necessary to work with your file.
Step 2: With our state-of-the-art PDF file editor, you're able to do more than merely fill in blanks. Edit away and make your docs seem great with custom textual content put in, or modify the original input to excellence - all comes with an ability to add any type of pictures and sign the PDF off.
This document will need particular data to be typed in, hence you need to take the time to type in what's asked:
1. When submitting the Dpr Form 39 030, ensure to complete all of the essential blanks in their associated form section. This will help speed up the work, making it possible for your details to be processed without delay and accurately.
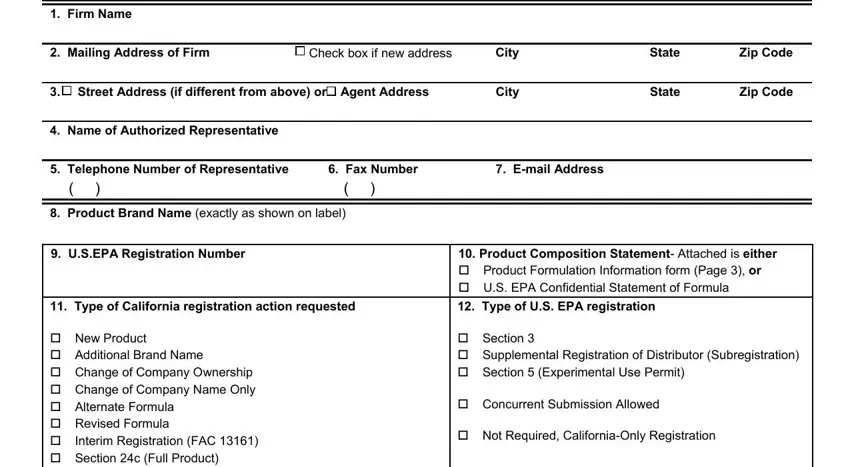
2. Immediately after the previous section is completed, go to type in the suitable information in all these - cid New Product cid Additional, Density, Liquid Product Lbs per Gallon, Solid Product Lbs per Cubic Foot, I certify under penalty of perjury, Signature of Authorized, Type or Print NameTitle, Date Signed, Mail completed application to For, DPR USE ONLY, and RC Fee RC Date Date Received.
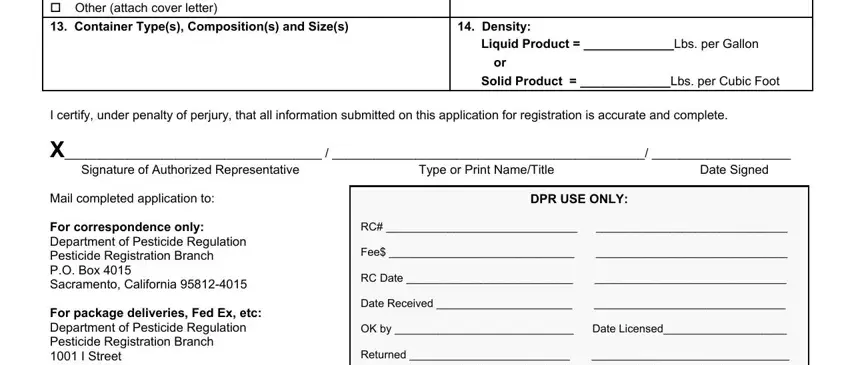
Be really mindful while filling in Solid Product Lbs per Cubic Foot and cid New Product cid Additional, because this is the section where most people make mistakes.
3. Within this stage, have a look at DPR FORM Rev Page of, Product Characterization, Brand Name EPA Reg No, Type of pesticide Check one or, cid Adjuvant inc water, cid Defoliant, modifiers, cid Algaecide, cid Antifoulant, cid Antimicrobial, cid Avicide, cid Bactericide Bacteriostat, cid Desiccant, cid DisinfectantSanitizer, and cid Fertilizer. All these should be filled in with greatest precision.

4. This specific paragraph arrives with the next few fields to look at: cid Attached eg collar, cid Filtration System, eartag, cid Bait, cid Broadcast, cid Fog, cid Fumigation, cid Injection, cid Chemigation or Drip, other than soil, cid Coating ie Seed, cid Paint or Coating, chisel or work into soil, cid Water Application, and cid Spray.

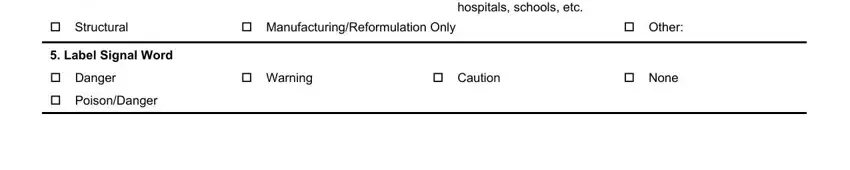
5. While you get close to the completion of your document, there are several more requirements that must be satisfied. Mainly, Use of Pesticide Check all that, cid HouseholdHome Garden cid, cid Industrial End Use, hospitals schools etc, cid Structural, cid ManufacturingReformulation Only, cid Other, Label Signal Word, cid Danger, cid PoisonDanger, cid Warning, cid Caution, and cid None must all be done.
Step 3: As soon as you have reviewed the details in the document, click "Done" to conclude your form. Sign up with us right now and immediately obtain Dpr Form 39 030, all set for downloading. All alterations you make are kept , allowing you to change the pdf later on as required. FormsPal guarantees your data privacy with a protected system that never records or shares any kind of personal data involved in the process. Be confident knowing your documents are kept safe each time you use our tools!