The public reporting burden for this collection of information is estimated to average 1.0 hour per response, including familiarization with the form, organizing the necessary information, and completing the form. Send any comments regarding the burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to: Chief, Information Policy Branch, 2136, U.S. Environmental Protection Agency, 401 M Street, S.W., Washington, D.C. 20460.
Instructions
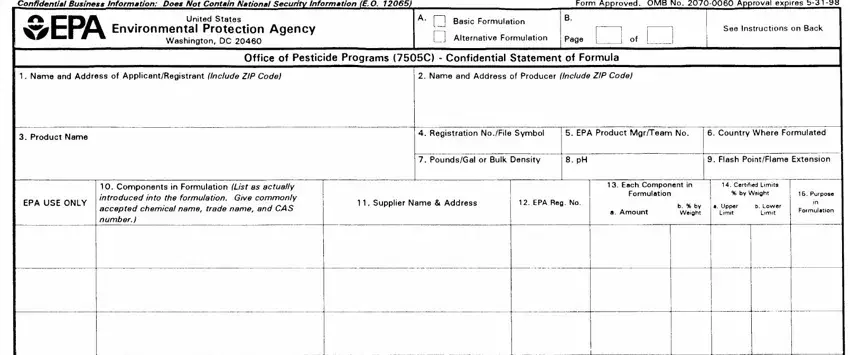
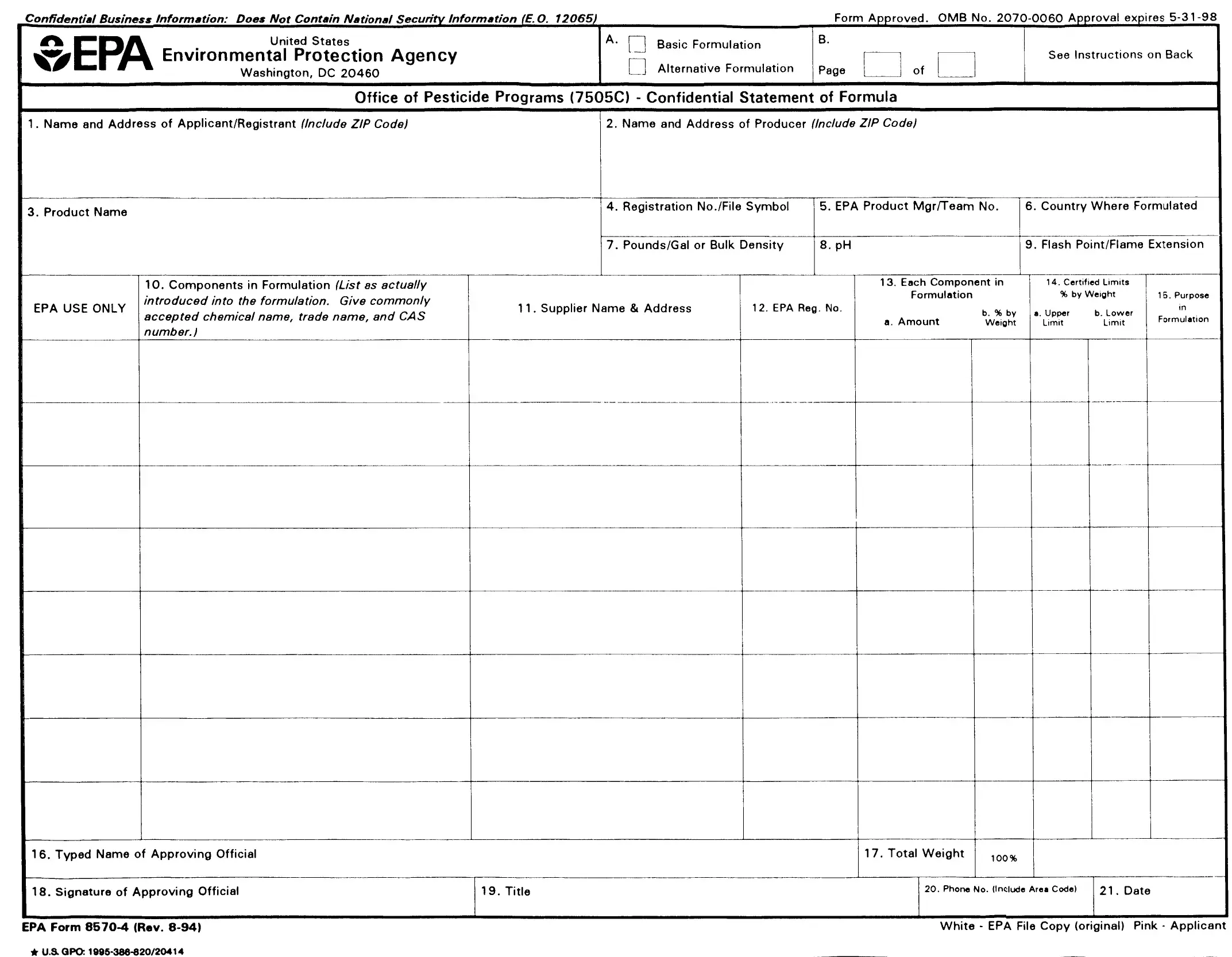
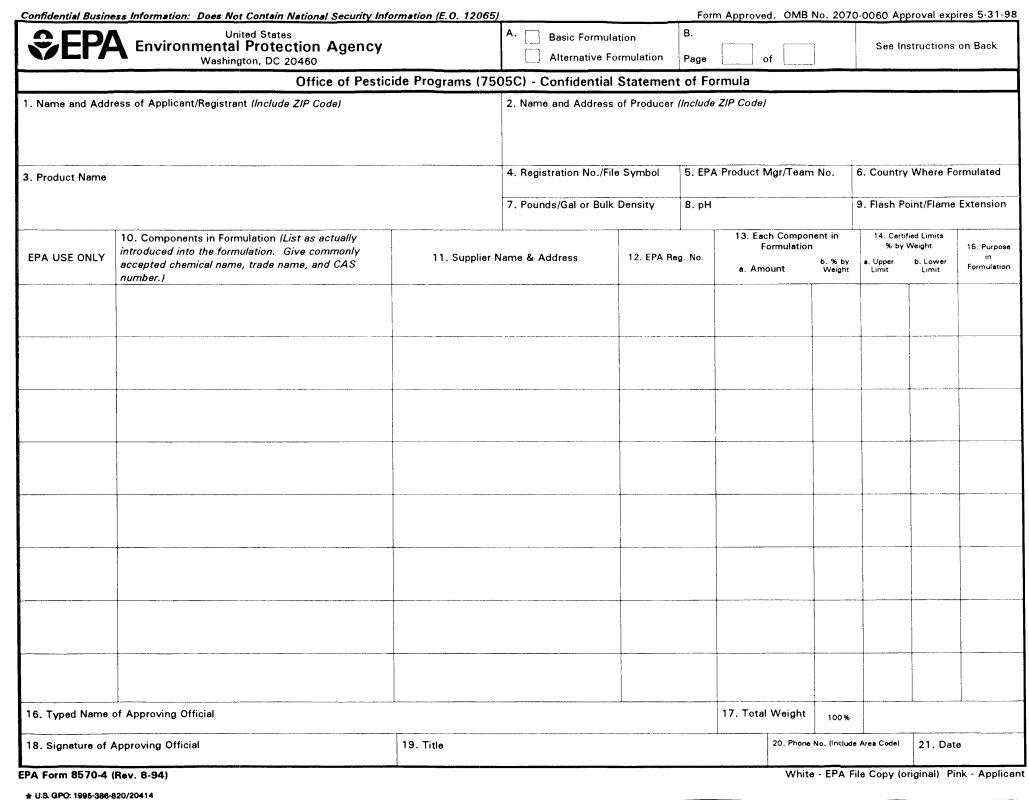
The complete chemical composition of each pesticide must be known so it can he evaluated for registration under the Federal Insecticide, Fungicide, and Rodenticide Act, as amended.
This form is designed for reporting the ingredients used in the formulation of a pesticide product. It must be completed and submitted with each application for new registration of a pesticide and application for amended registration if the revision involves a formula change.
Block A: Check the appropriate action for which you are submitting the form.
Block B: Number all pages consecutively. Enter on each page the total number of pages submitted. If more than one page is required, number them "1 of 2", "2 of2", "3 of 3", etc.
1.Name and Address of Applicant/Registrant: Enter the name and address of your firm or authorized agent.
2.Name and Address of Producer: Specify the name of the producer and the address of the site where this product will be produced.
3.Product Name: Specify the complete name of this pesticide product as it will appear on the label. This name must be the same as that which appears on the application form.
5.EPA Product Manager/Team Number: Enter the name and team number of the EPA Product Manager assigned to this product, if known.
6.Country Where Formulated: Specify the country where this product is formulated
7.Weight per Gallon/Bulk Density: For a liquid product specify pounds per gallon of formulated product. For a powder or granular product, enter the
hulk density of formulated product (as used). Enter weight per unit if the product is produced as a tablet, briquette, or other uniformly shaped product
8.pH: Enter the pH of aqueous formulations and products which are either dispersible or soluble in water. If not applicable enter "N/A".
9.Flash Point/Flame Extension: Specify the flash point as determined by the regulations for pressurized products and/or products known or suspected to burn. State the results of the flame extension test for pressurized products including positive flashbacks.
10.Components in Formulation: List as actually introduced into the formulation. For each component in your formulation, provide the product
name, commonly accepted chemical, the trade name, and the Chemical Abstract (CAS) number for each identifiable ingredient present in the product. CAS numbers may be obtained from the Chemical Abstract Service of the American Chemical Society, Columbus, OH. For each original and alternate source of each active ingredient in the product, indicate the percent purity of the manufacturing use product, technical product, or other source of active ingredient. If one or more components will be obtained from more than one source, enter all alternate sources and all alternate EPA Reg. Nos. in blocks 10, 11, and 12 or on a separate attachment.
Attention: (Special Instructions for Columns 10, 13, and 14) Any impurities
greater than or equal to 0.1% (or less than 0.1% if the impurity is toxicologically significant) which are associated with the active ingredient(s) of n technical grade (manufacturing or reformulating use) product or an end
use product produced by an integrated formulations system should also be listed in column 10, and the corresponding amount, percent by weight, and
upper certified limits in columns I3 and 14.
11.Supplier Name and Address: Provide the name and address of the supplier of each component in the formulation. If one or more components will be obtained from more than one source, specify the names addresses of the alternate sources also.
data required under 40 CFR Part 158.
13.Each Component in Formulation a. Amount: Specify the quantity of each component as actually introduced into the formulation. Units (e.g., pounds, grams, gallons, liters) should be expressed as used in the formulation. If the quantity is a liquid measure, enter the volume and the
specific gravity or the pounds per gallon of the component.
b. Percent by Weight: Specify the weight percentage of each component in your formulation. Check Your Calculations. Note that the weight percentage in many cases will not agree with that shown on the label ingredient statement where the weight percentage of the per active ingredient(s) must he declared. Attention: Producers of Microbial Products: Special Instructions for
Column 13b.) Please state the percent of active ingredient in British International Units (BIUs). International Toxic Units (ITUs). Polyhedral Inclusion Bodies (PIBs)(viruses), Colony Forming Units (CFUs)(Fungi), as
appropriate, and include an equivalent statement of active ingredient per milligram, ounce, pound, etc. of product (e.g., a 50% active Bacillus
thuringiensis product may have an equivalency value of 1.59 million Aedis aegypti ITU per pound of product.
14.Certified Limits: These limits are to be set based on representative sampling and chemicnl analysis (i.e., quality control) of the product.
a. Upper Limit: Specify the maximum percentage of each active ingredient, intentionally added inert ingredient, and any impurities greater than 0.1% to he permitted in the product.
b. Lower Limit: Specify the minimum percentage of each active ingredient and intentionally added inert ingredient to he permitted in the product.
15.Purpose In Formulation: Specify the purpose of each ingredient both active and inert. (For example, disinfectant, herbicide, synergist surfactant, defoamer, sequestrant, etc.) If space is insufficient, abbreviate.
16.Typed Name of Approving Offirial: Complete this item for identification of individual to he contacted if necessary
17.Total Weight: Specify the total weight of the batch (column 13a.) 18-21: Complete these items for identification of individual to be contacted if necessary.