Should you intend to fill out Epa Form 8570 27, it's not necessary to install any sort of applications - just use our online PDF editor. Our editor is consistently evolving to grant the best user experience achievable, and that's because of our resolve for continual development and listening closely to user feedback. Should you be seeking to begin, here is what it will require:
Step 1: Click the "Get Form" button above. It is going to open our pdf editor so that you could start filling in your form.
Step 2: With our state-of-the-art PDF editing tool, you may accomplish more than just fill out blank form fields. Try all the features and make your docs appear sublime with custom textual content put in, or adjust the file's original content to perfection - all comes with the capability to add any kind of pictures and sign the file off.
Be attentive while completing this pdf. Make sure that all necessary areas are filled out properly.
1. While filling in the Epa Form 8570 27, make certain to incorporate all of the important blanks in the relevant form section. It will help facilitate the work, enabling your details to be processed quickly and appropriately.

2. Just after completing the previous step, go to the subsequent part and complete all required details in all these fields - cid A An accurate Confidential, That formula statement indicates, paragraph, cid B The Confidential Statement, accurate and contains the, The following active ingredients, Active Ingredient, Product Name, Registration Number, Source, Signature, Name and Title, and Date.
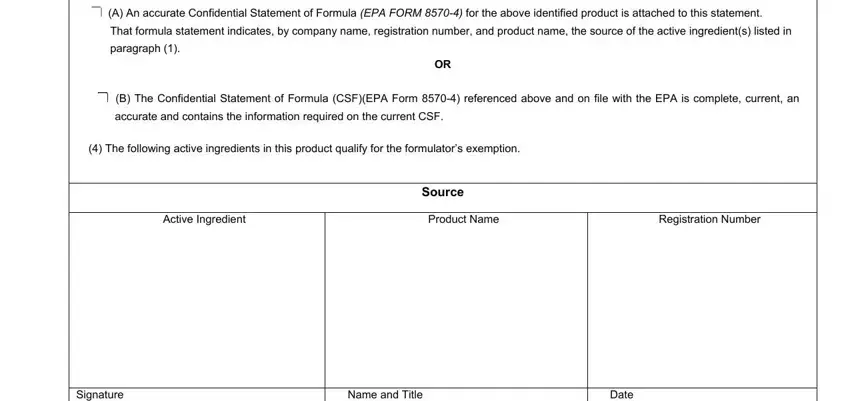
3. Within this part, check out Signature, Name and Title, Date, EPA Form Rev, and Copy EPA Copy Applicant copy. Each of these are required to be filled out with utmost accuracy.

Be very careful when completing Copy EPA Copy Applicant copy and Name and Title, as this is where a lot of people make mistakes.
Step 3: Make sure the details are correct and then just click "Done" to proceed further. After creating a7-day free trial account at FormsPal, it will be possible to download Epa Form 8570 27 or send it via email directly. The PDF document will also be at your disposal via your personal account with your every edit. Here at FormsPal.com, we do our utmost to be certain that your details are kept protected.