STATE OF CALIFORNIA |
DEPARTMENT OF CORRECTIONS AND REHABILITATION |
EMPLOYEE TUBERCULIN SKIN TEST (TST) AND EVALUATION |
|
CDCR 7336 (Rev. 07/15) |
Page 1 of 3 |
CONFIDENTIAL EMPLOYEE MEDICAL INFORMATION
INSTRUCTIONS: Tuberculosis (TB) screening must be performed by a licensed health care provider whose legally authorized scope of practice allows him/her to conduct medical examinations and/or the MantouxTB Skin Test (TST) in accordance with the recommendations of the Centers for Disease Control and Prevention to determine if a person has TB infection or disease.
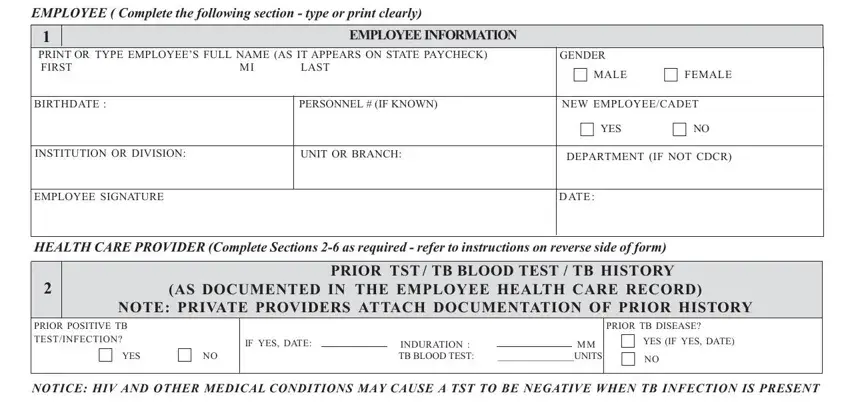
EMPLOYEE ( Complete the following section - type or print clearly)
PRINT OR TYPE EMPLOYEE’S FULL NAME (AS IT APPEARS ON STATE PAYCHECK) |
GENDER |
|
FIRST |
MI |
LAST |
MALE |
FEMALE |
|
|
|
|
|
|
|
BIRTHDATE : |
|
PERSONNEL # (IF KNOWN) |
NEW EMPLOYEE/CADET |
|
|
|
YES |
NO |
|
|
|
|
INSTITUTION OR DIVISION: |
|
UNIT OR BRANCH: |
DEPARTMENT (IF NOT CDCR) |
HEALTH CARE PROVIDER (Complete Sections 2-6 as required - refer to instructions on reverse side of form)
2 |
|
|
|
PRIOR TST / TB BLOOD TEST / TB HISTORY |
|
(AS DOCUMENTED IN THE EMPLOYEE HEALTH CARE RECORD) |
|
NOTE: PRIVATE PROVIDERS ATTACH DOCUMENTATION OF PRIOR HISTORY |
PRIOR POSITIVE TB |
|
|
|
|
|
|
PRIOR TB DISEASE? |
TEST/INFECTION? |
|
IF YES, DATE: |
|
INDURATION : |
|
MM |
YES (IF YES, DATE) |
|
YES |
NO |
|
|
TB BLOOD TEST: |
________________UNITS |
NO |
NOTICE: HIV AND OTHER MEDICAL CONDITIONS MAY CAUSE A TST TO BE NEGATIVE WHEN TB INFECTION IS PRESENT
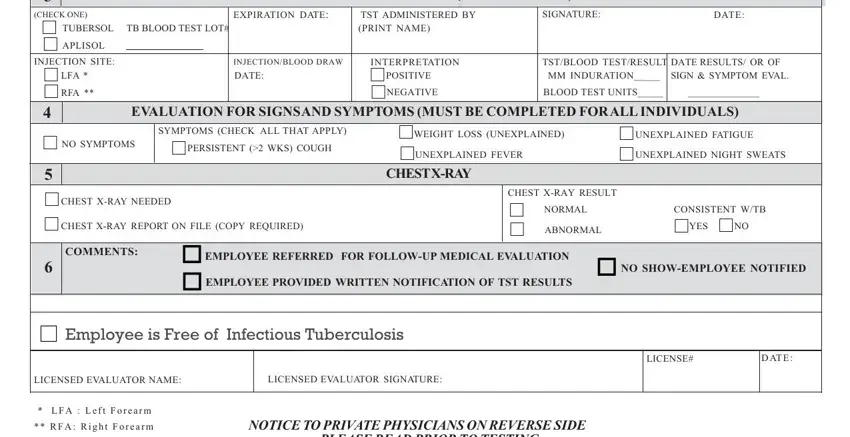
TST ADMINISTRATION (5TU/ 0.1 milliliter)/BLOOD TEST
(CHECK ONE) |
EXPIRATION DATE: |
TST ADMINISTERED BY |
SIGNATURE: |
DATE: |
TUBERSOL TB BLOOD TEST LOT# |
|
(PRINT NAME) |
|
|
APLISOL |
|
|
|
|
|
|
|
|
|
|
|
INJECTION SITE: |
INJECTION/BLOOD DRAW |
INTERPRETATION |
TST/BLOOD TEST/RESULT |
DATE RESULTS/ OR OF |
LFA * |
DATE: |
POSITIVE |
MM INDURATION_____ |
SIGN & SYMPTOM EVAL. |
RFA ** |
|
NEGATIVE |
BLOOD TEST UNITS_____ |
______________ |
4EVALUATION FOR SIGNSAND SYMPTOMS (MUST BE COMPLETED FORALL INDIVIDUALS)
|
|
|
|
|
|
SYMPTOMS (CHECK ALL THAT APPLY) |
|
|
|
NO SYMPTOMS |
PERSISTENT (>2 WKS) COUGH |
WEIGHT LOSS (UNEXPLAINED) |
|
UNEXPLAINED FATIGUE |
UNEXPLAINED FEVER |
UNEXPLAINED NIGHT SWEATS |
|
CHEST X-RAY NEEDED |
|
CHEST X-RAY RESULT |
|
|
|
|
NORMAL |
|
CONSISTENT W/TB |
|
CHEST X-RAY REPORT ON FILE (COPY REQUIRED) |
|
|
ABNORMAL |
YES NO |
|
6 |
COMMENTS: |
EMPLOYEE REFERRED FOR FOLLOW-UP MEDICAL EVALUATION |
NO SHOW-EMPLOYEE NOTIFIED |
|
|
EMPLOYEE PROVIDED WRITTEN NOTIFICATION OF TST RESULTS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Employee is Free of Infectious Tuberculosis |
|
|
|
|
LICENSED EVALUATOR SIGNATURE:
*LFA : Left Forearm ** RFA: Right Forearm
NOTICE TO PRIVATE PHYSICIANS ON REVERSE SIDE PLEASE READ PRIOR TO TESTING
Page 2 of 3
STATE OF CALIFORNIADEPARTMENT OF CORRECTIONS AND REHABILITATION
EMPLOYEE TUBERCULIN SKIN TEST (TST) AND EVALUATION
CDCR 7336 (Rev. 07/15)
NOTICE TO PRIVATE PHYSICIANS
CONFIDENTIAL EMPLOYMENT MEDICAL INFORMATION
THE CALIFORNIA PENAL CODE, SECTION 6006 et seq., REQUIRES ALL CALIFORNIA DEPARTMENT OF CORRECTIONS AND REHABILITATION (CDCR) employees and certain other individuals to have an initial, annual, and as medically necessary Mantoux Tuberculin Skin Test (TST) or evaluation. The testing must occur as instructed below. The employee must provide the results of the TST and/or evaluation on the REQUIRED form: the Employee Annual Tuberculin Skin Test (TST) and Evaluation, CDCR 7336.
DEFINITIONS:
INDURATION: Swelling or raised skin. Note: the presence of erythema is NOT indicative of a TST reaction; only the induration is measured.
MANTOUX TST: Intradermal injection of 0.1 milliliters (ml) of Purified Protein Derivative, 5 Tuberculin Units (TU).
PRIOR TST: A Mantoux TST in which clearly documented and dated results are available in millimeters (mm).
NEGATIVE TST RESULT: Induration of less than (<) 10 mm if new, or < 5 mm, if contact or known immunocompromised.
POSITIVE TST RESULT: Induration equal to or greater than (>) 10 mm, OR > 5 mm if contact or known immunocompromised.
INSTRUCTIONS: EMPLOYEE
1.Complete all of the items in SECTION 1 - All Boxes Must Be Completely Filled In.
•Be sure the information you provide is accurate and complete.
•The health care provider(s) (HCP) administering and evaluating the TST, including the exam for TB signs and
symptoms, must sign and date the appropriate blocks.
•Advise the HCP to follow the steps below when completing SECTION 2 through SECTION 6.
•If a chest x-ray (CXR) is needed, you must submit a copy of the CXR report with this form to be placed in your health record.
•Submit the completed form (Employee Tuberculin Skin Test (TST) and Evaluation, CDCR 7336), in a sealed envelope, as instructed by your supervisor/TB coordinator.
INSTRUCTIONS: HEALTH CARE PROVIDER - All Boxes Must Be Completely Filled In.
SECTION 2: If prior test TST results are available, the employee or HCP must provide written documentation including the patient’s name, date test was administered, and reaction in mm or IU. Document this in SECTION 2. If documented results are:
•NEGATIVE and more than 30 days old, proceed to Section 3.
•NEGATIVE and less than 30 days old, proceed to Section 4.
•POSITIVE on any date: proceed to Section 4. Must also complete Section 5.
If there are no appropriately documented prior TST or TB blood test results, go to the instructions for Section #3.
SECTION 3: Administer a new TST or TB blood test, and document results in SECTION 3. NOTE: The HCP administering the TST (SECTION 3), and the HCP evaluating the TST (SECTION 6), must sign in the appropriate blocks. If the TST or TB blood test results are:
•NEGATIVE, complete Section 4. Evaluator must sign and date under Section 6.
•POSITIVE, proceed to Section 4. Must also complete Section 5. Evaluator must sign and date under Section 6.
If an individual claims to have a prior positive TB testST, but cannot provide appropriate documentation, a TST or TB blood test must still be administered.
This is not medically contraindicated. However, if there are still questions, although this is not a CDCR procedure, it has been found useful to administer a diluted TST: dilute 0.2 cc of the standard 5 TU/0.1cc solution with 0.8 cc of sterile saline, then use
0.1of this solution to administer a TST. If the results are positive, no further testing is necessary, proceed as directed below for positive TST’s. If the results are negative, proceed with a standard TST.
If the administered or documented TST or TB blood testshows a NEGATIVE result, the employee probably does not have TB infection. Factors affecting the immune system, pregnancy, or recent TB infection may cause a false negative TST or TB blood test reaction, even when TB disease exists, but
CDCR HCPs CANNOT ASK CDCR EMPLOYEES ABOUT NON TB HEALTH HISTORY, INCLUDING
IMMUNOSUPPRESSIVE CONDITIONS
If the TB test TST indicates a POSITIVE reaction, further medical evaluation and a CXR are needed to rule out active TB disease.
Page 3 of 3
STATE OF CALIFORNIADEPARTMENT OF CORRECTIONS AND REHABILITATION
EMPLOYEE TUBERCULIN SKIN TEST (TST) AND EVALUATION
CDCR 7336 (Rev. 07/15)
•Complete SECTIONS 4, 5 AND 6. The HCP evaluating for TB signs and symptoms, must sign and date the form in the space provided at the bottom of the form (SECTION 6).
•Give a copy of the CXR report, if a CXR is taken, to the employee for the CDCR records.
The space identified as “DATE TST READ OR OF SIGNS & SYMPTOMS EXAM” refers to date that the employee’s TB status is determined.
•After evaluation and/or treatment the CDCR 7336 is completed.
•Give the completed CDCR 7336 and the CXR report to the employee.
SECTION 4: Complete evaluation for all employees, regardless of TB testTST result, for TB signs and symptoms; 3 or more positives warrant special concern.
SECTION 5: To be completed for individuals with a documented prior or newly significant TST or TB blood test. Attach copy of CXR report.
SECTION 6: Comments as necessary. Evaluator (Physician and Surgeon or a licensed designee) must sign and date the form.
The Centers for Disease Control and Prevention and the California Tuberculosis Controllers Association recommend the
following:
1.Tine test is NOT an acceptable skin test to determine exposure to the TB bacillus.
2.CXR is an unacceptable screening method for detecting TB infection.
3.The only acceptable screening method(s) for detecting TB infection are TB screening tests that are licensed by the Federal Food and Drug Administration (FDA) and recommended by the Centers for Disease Control (CDC).
4.The process for administering, evaluation, and documenting the Mantoux TST are:
a)Must be given intradermally.
b)0.1 ml (s) of 5 TU Purified Protein Derivative must be used.
c)The test must be interpreted by a qualified HCP.
d)Results must be documented/reported in mm(s) of induration.
DISTRIBUTION: WHITE- HCSD PUBLIC HEALTH SECTION, YELLOW- EMPLOYEE MEDICAL FILE, PINK- EMPLOYEE