In navigating the complex terrain of public health management, particularly in the context of infectious disease control, the Epid 200 form serves as a crucial instrument for professionals within the Kentucky Department for Public Health. Revised in June 2010, this form embodies a comprehensive data collection tool used to report various contagious illnesses that may pose significant health risks to the community. The form meticulously gathers demographic data of the patient, including name, date of birth, contact information, and ethnic origin, providing a clear patient snapshot. It further delves into the specifics of the disease in question—naming the disease, pinpointing the onset and diagnosis dates, listing symptoms, and capturing hospitalization details if applicable. For diseases transmitted through sexual contact, the form expands to encompass additional information pertinent to case detection, disease stage, and treatment rendered. Crucially, the EPID 200 form operates within a regulatory framework established by Kentucky state law 902 KAR 2:020, mandating health professionals to promptly report a delineated list of diseases to local health departments or directly to the Kentucky Department for Public Health. This regulation underscores the urgency of reporting certain diseases, ranging from those requiring immediate telephone notification, such as outbreaks indicative of a newly recognized infectious agent or bioterrorism acts, to others necessitating reportage within specified time frames. The structured yet detailed format of the EPID 200 form underscores Kentucky's proactive stance in disease surveillance and control, ensuring timely and organized reporting that is vital for initiating appropriate public health responses.
| Question | Answer |
|---|---|
| Form Name | Form Epid 200 |
| Form Length | 2 pages |
| Fillable? | No |
| Fillable fields | 0 |
| Avg. time to fill out | 30 sec |
| Other names | KAR, kentucky reportable disease form 2020, Neuroinvasive, kentucky reportable disease form |
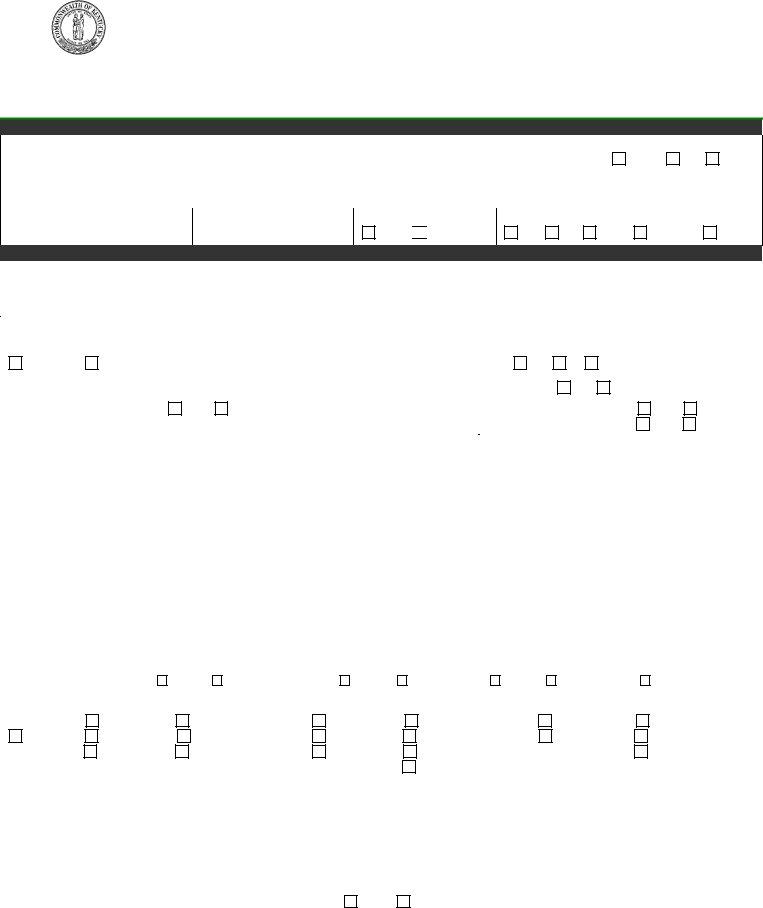
EPID 200 (Rev Jun 2010)
Kentucky Reportable Disease Form
Department for Public Health
Division of Epidemiology and Health Planning
275 East Main St., Mailstop
Frankfort, KY
Disease Name_____________________
Mail Form to Local Health Department
DEMOGRAPHIC DATA
Patient’s Last Name |
First |
M.I. |
Date of Birth |
|
Age |
|
Gender |
|
|
|
|
|
/ |
/ |
|
|
M |
F |
Unk |
|
|
|
|
|
|
|
|
||
Address |
City |
State |
|
|
Zip |
County of Residence |
|||
|
|
|
|
|
|
|
|
|
|
Phone Number
Patient ID Number
Ethnic Origin
His.
Race
W
B
A/PI
Am.Ind.
Other
DISEASE INFORMATION
Disease/Organism |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Date of Onset |
|
|
Date of Diagnosis |
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
/ |
|
|
/ |
|
|
|
/ |
/ |
|||
List Symptoms/Comments |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Highest Temperature |
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Days of Diarrhea |
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Hospitalized? |
|
|
|
|
|
|
Admission Date |
|
Discharge Date |
|
|
Died? |
|
|
|
|
Date of Death |
|||||||||||||
Yes |
No |
|
|
|
|
/ |
|
/ |
|
|
|
|
/ |
/ |
|
|
|
|
Yes |
No |
Unk |
|
/ |
/ |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Hospital Name: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Is Patient Pregnant? Yes |
No |
If yes, # wks_____ |
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
School/Daycare Associated? |
|
Yes |
No |
|
|
|
|
|
|
|
|
|
|
|
|
|
Outbreak Associated? |
|
Yes |
No |
||||||||||
Name of School/Daycare: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Food Handler? |
|
|
|
|
Yes |
No |
|||||
Person or Agency Completing form: |
|
|
|
|
|
|
|
|
|
|
|
|
Attending Physician: |
|
|
|
|
|
|
|||||||||||
Name: |
|
|
|
|
|
|
|
Agency: |
|
|
|
|
|
|
|
|
Name: |
|
|
|
|
|
|
|
||||||
Address: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Address: |
|
|
|
|
|
|
|
||||
Phone: |
|
|
|
|
|
|
|
|
Date of Report: |
/ |
/ |
|
|
Phone: |
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
LABORATORY INFORMATION |
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
Date |
|
Name or Type of Test |
Name of Laboratory |
|
|
Specimen Source |
|
|
Results |
|
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
|
|
ADDITIONAL INFORMATION FOR SEXUALLY TRANSMITTED DISEASES ONLY |
|
|
|
|||||||||||||||||||||||||
Method of case detection: |
Prenatal |
Community & Screening Delivery |
Instit. Screening |
|
Reactor |
Provider Report |
|
Volunteer |
|
|||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Disease: |
|
Stage |
|
|
|
|
|
|
Disease: |
|
Site: (Check all that apply) |
|
|
|
|
Resistance: |
||||||||||||||
|
Primary (lesion) |
Secondary (symptoms) |
|
Gonorrhea |
|
Genital, uncomplicated |
Ophthalmic |
|
Penicillin |
|
||||||||||||||||||||
Syphilis |
Early Latent |
|
Late Latent |
|
|
|
Chlamydia |
|
Pharyngeal |
|
|
|
|
|
PID/Acute |
|
|
|
|
Tetracycline |
||||||||||
|
Congenital |
|
Other |
|
|
|
Chancroid |
|
Anorectal |
|
|
|
|
|
Salpingitis |
|
Other ___________ |
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other___________________ |
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
Date of spec. |
Laboratory Name |
|
|
Type of Test |
|
Results |
|
Treatment Date |
|
Medication |
|
|
|
|
Dose |
|
||||||||||||||
Collection |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
If syphilis, was previous treatment given for this infection? |
|
Yes |
|
No |
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
If yes, give approximate date and place_______________________________________________________________ |
|
|
|
|||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
902 KAR 2:020 requires health professionals to report the following diseases to the local health departments serving the jurisdiction in which the patient resides or to the Kentucky Department for Public Health (KDPH).
(Copies of 902 KAR 2:020 available upon request)
REPORT IMMEDIATELY by TELEPHONE to the Local Health Department or the KY Department for Public Health:
•Unexpected pattern of cases, suspected cases or deaths which may indicate a newly recognized infectious agent
•An outbreak, epidemic, related public health hazard or act of bioterrorism, such as SMALLPOX
Kentucky Department for Public Health in Frankfort
Telephone
SECURED FAX
REPORT WITHIN 24 HOURS
Anthrax |
Hansen’s disease |
Rubella |
Arboviral Disease* |
Hantavirus infection |
Rubella syndrome, congenital |
Neuroinvasive |
Hepatitis A |
Salmonellosis |
Listeriosis |
Shigellosis |
|
Botulism |
Measles |
Syphilis, primary, secondary, |
Brucellosis |
Meningococcal infections |
early latent or congenital |
Campylobacteriosis |
Pertussis |
Tetanus |
Cholera |
Plague |
Tularemia |
Cryptosporidiosis |
Poliomyelitis |
Typhoid Fever |
Diphtheria |
Psittacosis |
Vibrio parahaemolyticus |
E. coli shiga toxin positive (STEC) |
Q Fever |
Vibrio vulnificus |
Haemophilus influenzae |
Rabies, animal |
Yellow Fever |
invasive disease |
Rabies, human |
|
REPORT WITHIN ONE (1) BUSINESS DAY
Foodborne outbreak |
Hepatitis B, acute |
Toxic Shock Syndrome |
Hepatitis B infection in a |
Mumps |
Tuberculosis |
pregnant woman or child |
Streptococcal disease |
Waterborne outbreak |
born in or after 1992 |
invasive, Group A |
|
REPORT WITHIN FIVE (5) BUSINESS DAYS
AIDS |
HIV infection |
Chancroid |
Lead poisoning |
Chlamydia trachomatis |
Legionellosis |
infection |
Lyme disease |
Ehrlichiosis |
Lymphogranuloma venereum |
Gonorrhea |
Malaria |
Granuloma inguinale |
Rabies, post exposure |
Hepatitis C, acute |
prophylaxis |
Histoplasmosis |
|
Rocky Mountain spotted fever
Streptococcus pneumoniae,
Syphilis, other than primary, secondary, early latent or congenital
Toxoplasmosis
*Includes Eastern Equine, Western Equine, California group, St. Louis, Venezuelan and West Nile Viruses Influenza virus isolates are to be reported weekly by laboratories.
902 KAR 02:065 requires long term care facilities to report an outbreak (2 or more cases) of
All cases of HIV infections/AIDS are reportable to a separate surveillance system in accordance with KRS 211.180(1)b. To report a HIV/AIDS case call
DO NOT REPORT HIV/AIDS CASES ON THIS FORM.
Note: Animal bites shall be reported to local health departments within twelve (12) hours in accordance with KRS 258:065.