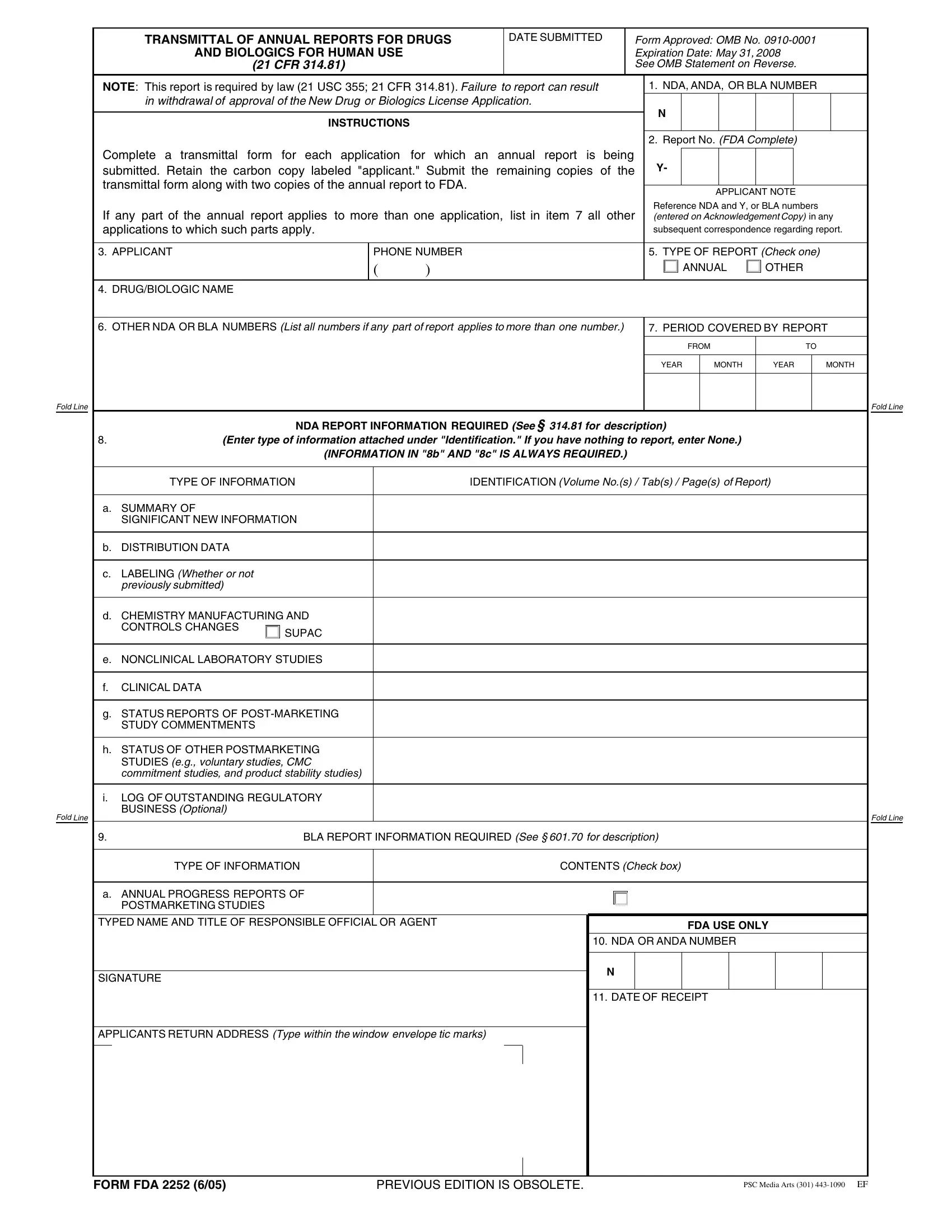
In case you desire to fill out fda form 2252 no download needed, you don't need to download and install any kind of applications - just give a try to our PDF tool. In order to make our tool better and simpler to work with, we constantly work on new features, taking into consideration feedback from our users. All it requires is several simple steps:
Step 1: Click the "Get Form" button at the top of this page to open our PDF tool.
Step 2: This editor lets you customize your PDF document in various ways. Enhance it by writing any text, correct existing content, and add a signature - all at your disposal!
Filling out this document generally requires care for details. Make sure that all required fields are completed correctly.
1. It's very important to fill out the fda form 2252 no download needed correctly, hence take care when working with the parts including all of these blanks:
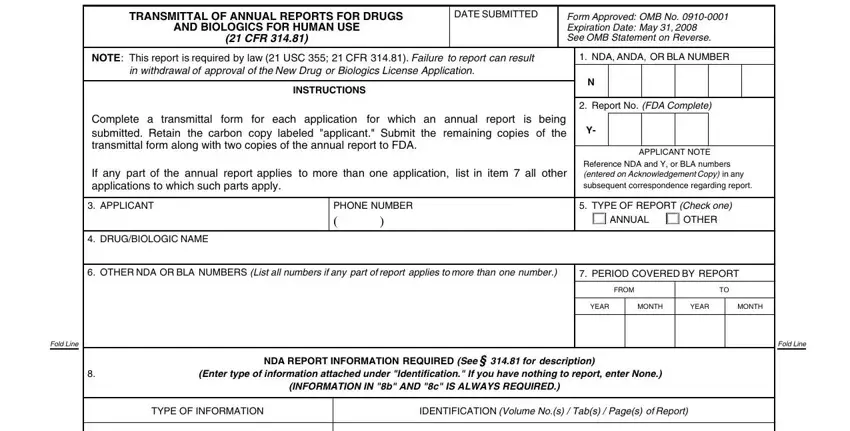
2. When the last part is completed, you have to put in the required particulars in a SUMMARY OF, SIGNIFICANT NEW INFORMATION, b DISTRIBUTION DATA, c LABELING Whether or not, previously submitted, d CHEMISTRY MANUFACTURING AND, CONTROLS CHANGES, SUPAC, e NONCLINICAL LABORATORY STUDIES, f CLINICAL DATA, g STATUS REPORTS OF POSTMARKETING, STUDY COMMENTMENTS, h STATUS OF OTHER POSTMARKETING, STUDIES eg voluntary studies CMC, and LOG OF OUTSTANDING REGULATORY so you're able to proceed to the next step.
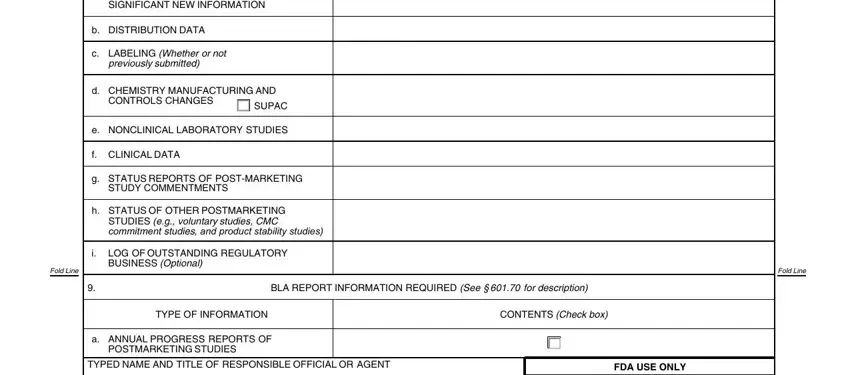
Always be really careful when filling in f CLINICAL DATA and g STATUS REPORTS OF POSTMARKETING, as this is the part where most people make mistakes.
3. Your next stage is going to be easy - fill in all of the empty fields in SIGNATURE, APPLICANTS RETURN ADDRESS Type, NDA OR ANDA NUMBER, DATE OF RECEIPT, FORM FDA, PREVIOUS EDITION IS OBSOLETE, and PSC Media Arts in order to complete this segment.
Step 3: Before getting to the next stage, ensure that blanks have been filled out right. As soon as you are satisfied with it, press “Done." Sign up with FormsPal today and immediately access fda form 2252 no download needed, set for download. All adjustments you make are preserved , helping you to change the document later on if required. FormsPal ensures your information privacy by having a secure method that in no way records or shares any sort of personal data involved in the process. Be confident knowing your documents are kept safe when you use our tools!