bpdr form 3486 can be filled in online easily. Just try FormsPal PDF tool to get the job done without delay. To have our editor on the leading edge of convenience, we strive to put into operation user-oriented capabilities and improvements regularly. We're always grateful for any feedback - help us with revampimg PDF editing. Here is what you would want to do to start:
Step 1: Access the form inside our editor by pressing the "Get Form Button" above on this page.
Step 2: Once you access the online editor, you will see the document all set to be completed. Besides filling in various blanks, you may also do other things with the form, that is putting on custom textual content, editing the initial text, adding graphics, placing your signature to the form, and more.
In order to complete this PDF form, be certain to enter the information you need in each blank:
1. It is advisable to complete the bpdr form 3486 properly, thus be attentive when filling in the areas containing all these blank fields:
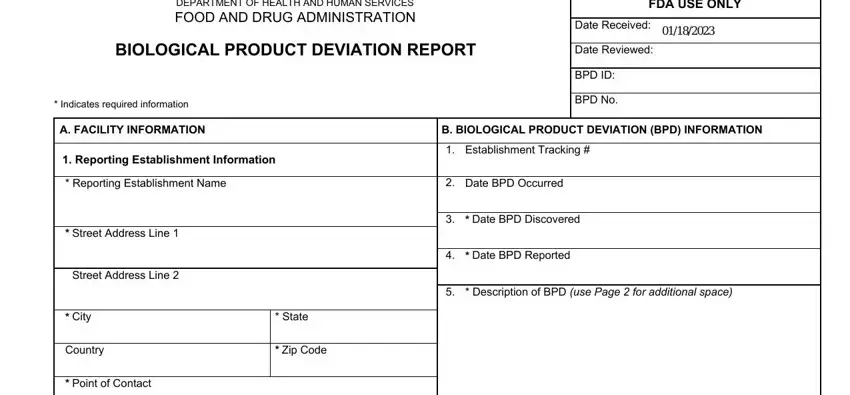
2. Soon after the last part is filled out, go on to enter the suitable information in all these: Telephone, Email, Reporting Establishment, FDA Registration, CLIA, If the BPD occurred somewhere, Description of Contributing, FollowUp use Page for additional, Establishment Name, Street Address Line, Street Address Line, City, Country, Please Enter the Character BPD, and State.

3. Completing FDA Registration, CLIA, FORM FDA, Please check the type, Blood, Continued on Page, of product, NonBlood, Continued on Page, Form Approved OMB No Expires, See PRA Statement on Page, Page of, and PSC Publishing Services is essential for the next step, make sure to fill them out in their entirety. Don't miss any details!

Always be extremely attentive when filling out Continued on Page and FORM FDA, since this is the part where a lot of people make mistakes.
4. It's time to fill out this fourth section! Here you'll get all these B DESCRIPTION OF BPD continued empty form fields to do.
5. The very last step to submit this PDF form is critical. Make sure that you fill out the mandatory blank fields, including B DESCRIPTION OF CONTRIBUTING, prior to using the file. Neglecting to do so could produce an incomplete and potentially nonvalid document!

Step 3: Be certain that the information is right and press "Done" to proceed further. Go for a free trial subscription at FormsPal and get immediate access to bpdr form 3486 - download or modify inside your personal account. FormsPal guarantees protected document completion devoid of personal information record-keeping or sharing. Feel comfortable knowing that your details are in good hands with us!








