You may fill out 3500B effectively with our online PDF editor. Our editor is consistently developing to deliver the very best user experience attainable, and that is thanks to our dedication to continual development and listening closely to feedback from customers. By taking a few easy steps, it is possible to start your PDF editing:
Step 1: Click the "Get Form" button at the top of this webpage to open our editor.
Step 2: The editor will let you modify your PDF form in various ways. Enhance it by writing any text, correct what's already in the PDF, and put in a signature - all within several mouse clicks!
Completing this document requires thoroughness. Ensure each and every field is completed properly.
1. The 3500B usually requires particular information to be entered. Be sure the subsequent blank fields are completed:
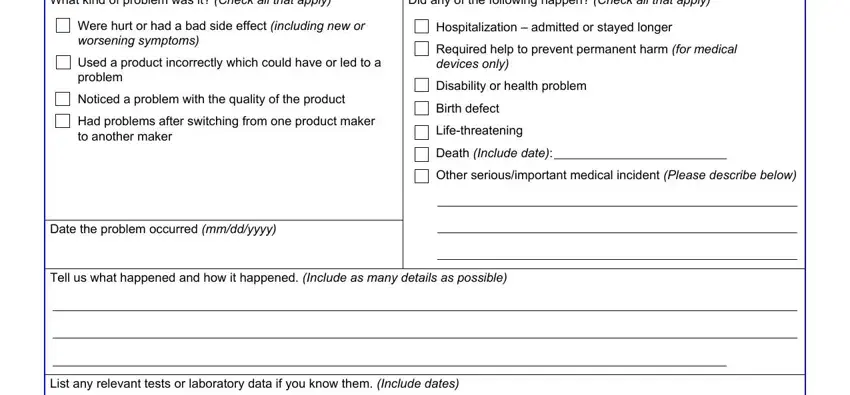
2. The third step is usually to complete the following fields: For a problem with a product, prescription or overthecounter, for example tendons ligaments and, nutrition products such as, formulas and medical foods, cosmetics or makeup products, foods including beverages and, Go to Section B, For a problem with a medical, any healthrelated test tool or, and Go to Section C Skip Section B.
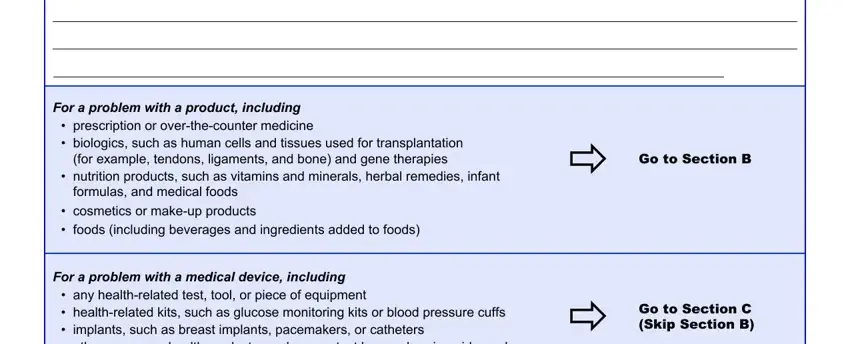
3. This third segment should also be rather straightforward, Name of the product as it appears, Name of the company that makes the, Expiration date mmddyyyy, Lot number, NDC number, Strength for example mg per mL, Quantity for example pills puffs, Frequency for example twice daily, How was it taken or used for, Date the person first started, Date the person stopped taking or, Did the problem stop after the, Yes, Why was the person using the, and Did the problem return if the - each one of these empty fields will have to be completed here.
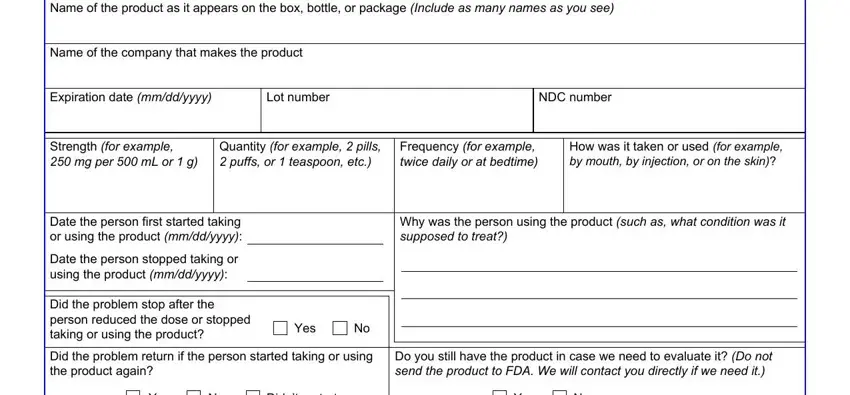
People who work with this form frequently make some mistakes while filling out Yes in this section. Remember to re-examine what you type in right here.
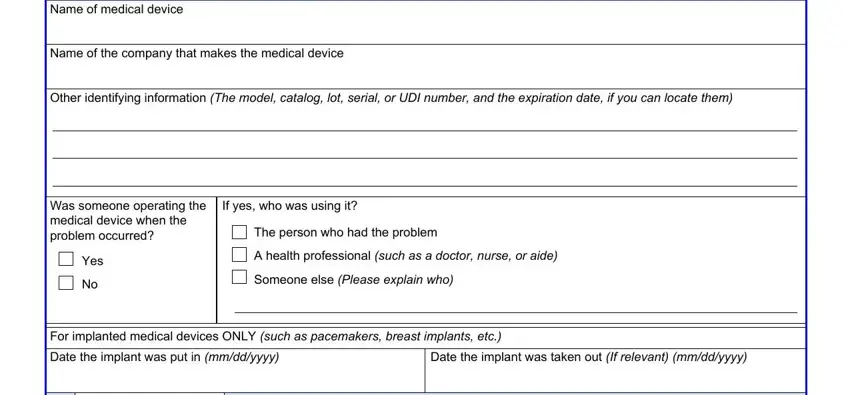
4. To go forward, the next section requires filling out several form blanks. Included in these are Name of medical device, Name of the company that makes the, Other identifying information The, Was someone operating the medical, If yes who was using it, The person who had the problem, Yes, A health professional such as a, Someone else Please explain who, For implanted medical devices ONLY, Date the implant was put in, and Date the implant was taken out If, which are integral to carrying on with this form.
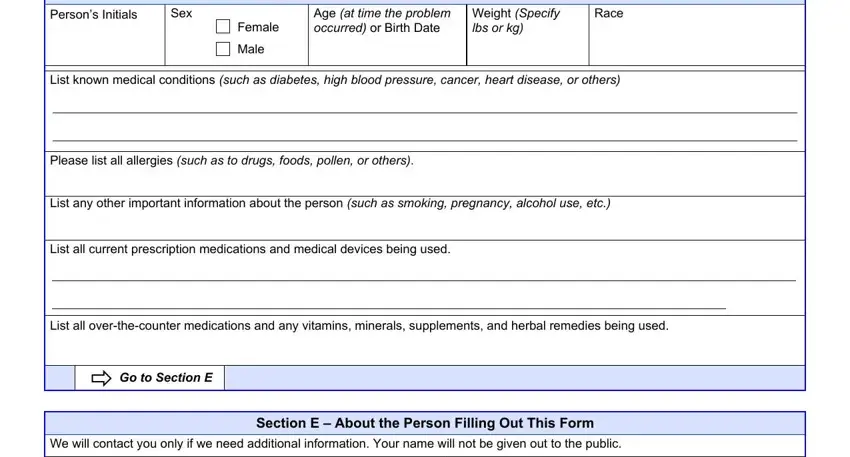
5. When you near the final sections of your file, you will find a couple extra requirements that must be fulfilled. In particular, Section D About the Person Who, Persons Initials, Sex, Female, Male, Age at time the problem occurred, Weight Specify lbs or kg, Race, List known medical conditions such, Please list all allergies such as, List any other important, List all current prescription, List all overthecounter, Go to Section E, and We will contact you only if we should all be done.
Step 3: After rereading your fields you've filled out, press "Done" and you're done and dusted! Make a free trial option at FormsPal and gain immediate access to 3500B - download or edit in your FormsPal cabinet. FormsPal is committed to the confidentiality of our users; we ensure that all personal data put into our editor stays confidential.