Dealing with PDF documents online is always a piece of cake with our PDF tool. You can fill in fda form 3613b here within minutes. To retain our editor on the forefront of efficiency, we aim to put into action user-oriented features and improvements on a regular basis. We are routinely pleased to get suggestions - join us in revolutionizing the way you work with PDF documents. In case you are looking to get started, here is what it will require:
Step 1: Press the "Get Form" button above. It is going to open our tool so you could begin completing your form.
Step 2: This editor lets you change your PDF form in many different ways. Modify it with personalized text, correct original content, and add a signature - all readily available!
As for the blanks of this specific PDF, this is what you want to do:
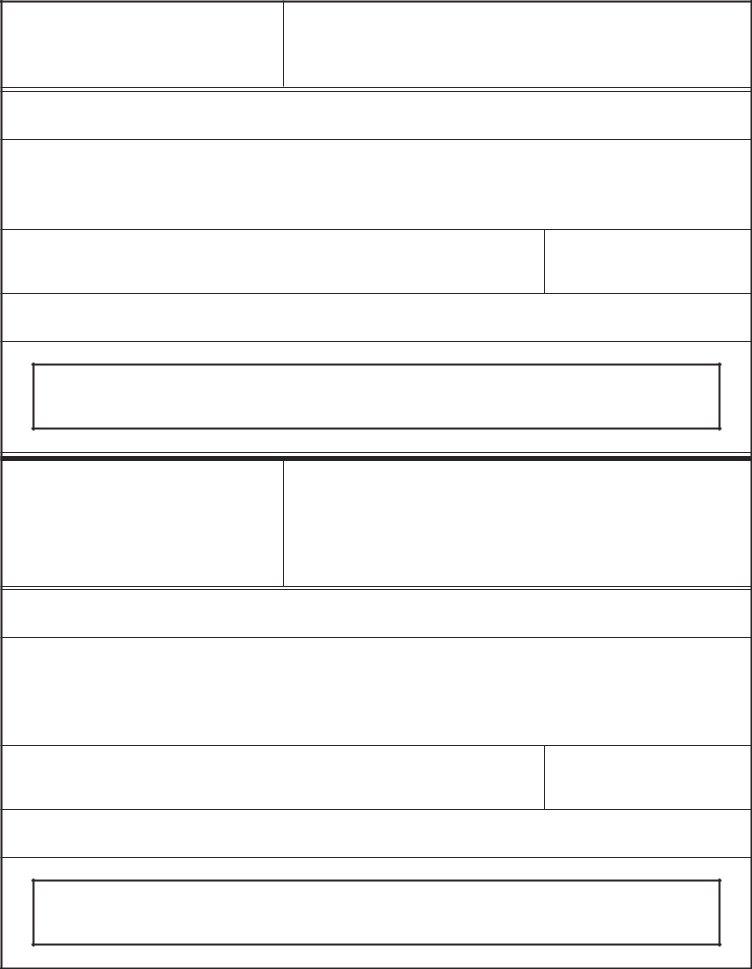
1. To start with, while filling out the fda form 3613b, beging with the area containing following blank fields:
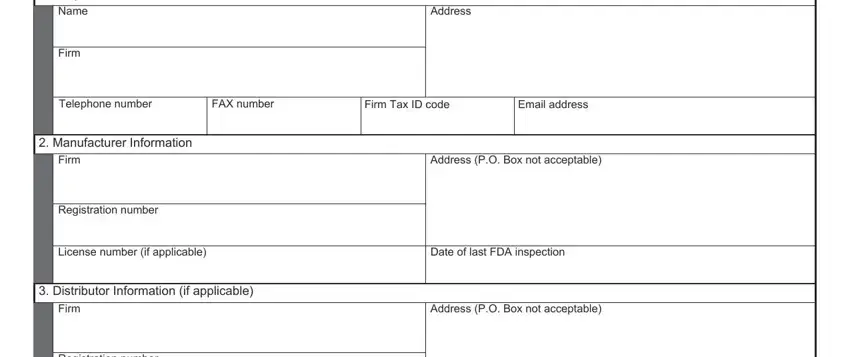
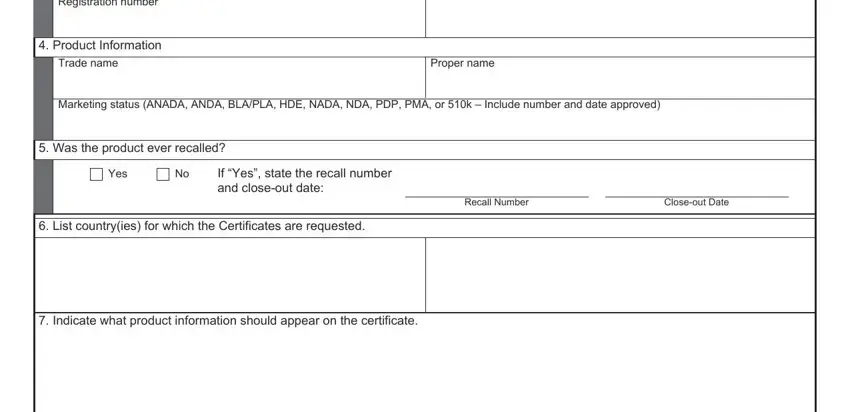
2. After completing this step, go to the next stage and complete the necessary particulars in these blanks - Registration number, Product Information, Trade name, Proper name, Marketing status ANADA ANDA BLAPLA, Was the product ever recalled, Yes, If Yes state the recall number and, List countryies for which the, Recall Number, Closeout Date, and Indicate what product information.
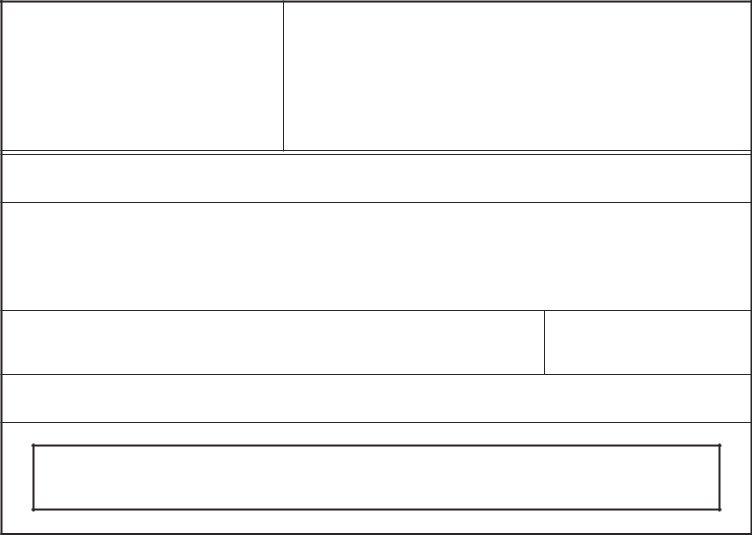
3. Completing Should the country destination be, Yes, Indicate the total number of, NOTE If the products being, Exporters Certification Statement, CBER instructions are on page, CDRH instructions are on page, CVM instructions are on page, FORM FDA, Page of, and PSC Publishing Services is essential for the next step, make sure to fill them out in their entirety. Don't miss any details!

4. This next section requires some additional information. Ensure you complete all the necessary fields - FIRM NAME, As the responsible official or, SIGNATURE, NAME AND TITLE, DATE, and Making or submitting false - to proceed further in your process!

A lot of people often make errors while filling out NAME AND TITLE in this area. Don't forget to reread what you enter here.
5. The last point to finish this document is pivotal. You need to fill out the displayed blank fields, and this includes FIRM NAME, As the responsible official or, SIGNATURE, NAME AND TITLE, DATE, Making or submitting false, FORM FDA, and Page of, prior to finalizing. Failing to accomplish that might end up in a flawed and possibly nonvalid paper!

Step 3: Soon after rereading your fields you have filled out, click "Done" and you are done and dusted! Get hold of your fda form 3613b the instant you subscribe to a 7-day free trial. Readily gain access to the pdf inside your FormsPal account page, with any edits and adjustments all synced! At FormsPal.com, we do everything we can to be sure that your information is kept secure.