By using the online editor for PDFs by FormsPal, you're able to complete or edit fda form 2877 here and now. To make our editor better and more convenient to utilize, we continuously design new features, considering suggestions from our users. Should you be seeking to begin, here is what it requires:
Step 1: Hit the orange "Get Form" button above. It's going to open our tool so you could start filling out your form.
Step 2: Using this advanced PDF editing tool, you are able to accomplish more than simply fill in forms. Try each of the functions and make your documents appear high-quality with custom text added, or modify the file's original input to perfection - all that supported by the capability to insert your own images and sign the document off.
This form will need specific info to be entered, thus you should definitely take whatever time to provide what is asked:
1. Fill out the fda form 2877 with a number of essential blanks. Consider all of the information you need and ensure not a single thing omitted!
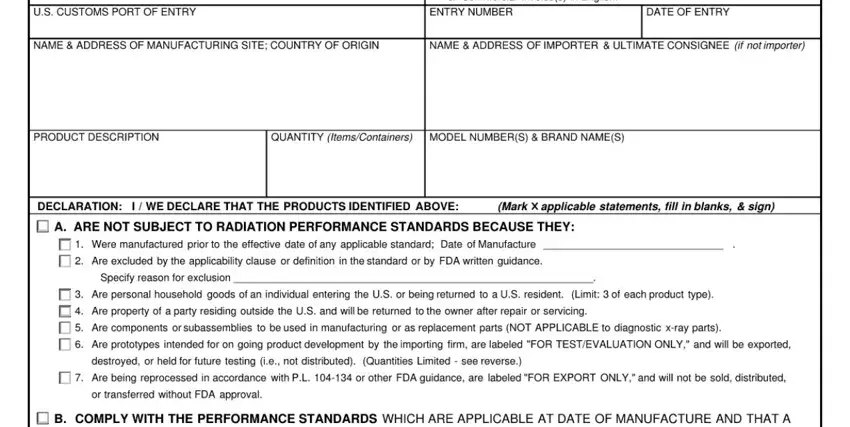
2. Now that this part is done, you're ready to include the required specifics in so you can move forward further.
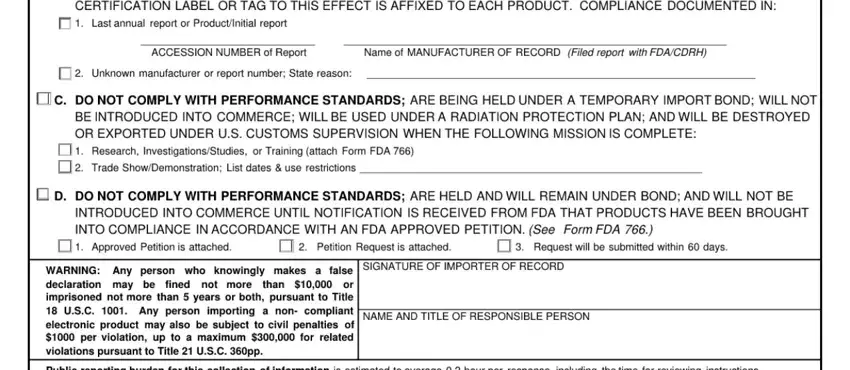
Regarding this field and next field, ensure that you review things here. The two of these are viewed as the most important fields in the document.
Step 3: Before moving forward, ensure that all blank fields were filled out as intended. As soon as you verify that it is correct, click “Done." Acquire your fda form 2877 the instant you subscribe to a 7-day free trial. Easily get access to the form from your personal account, with any modifications and changes being automatically kept! We do not share or sell the information that you type in while completing forms at our website.



