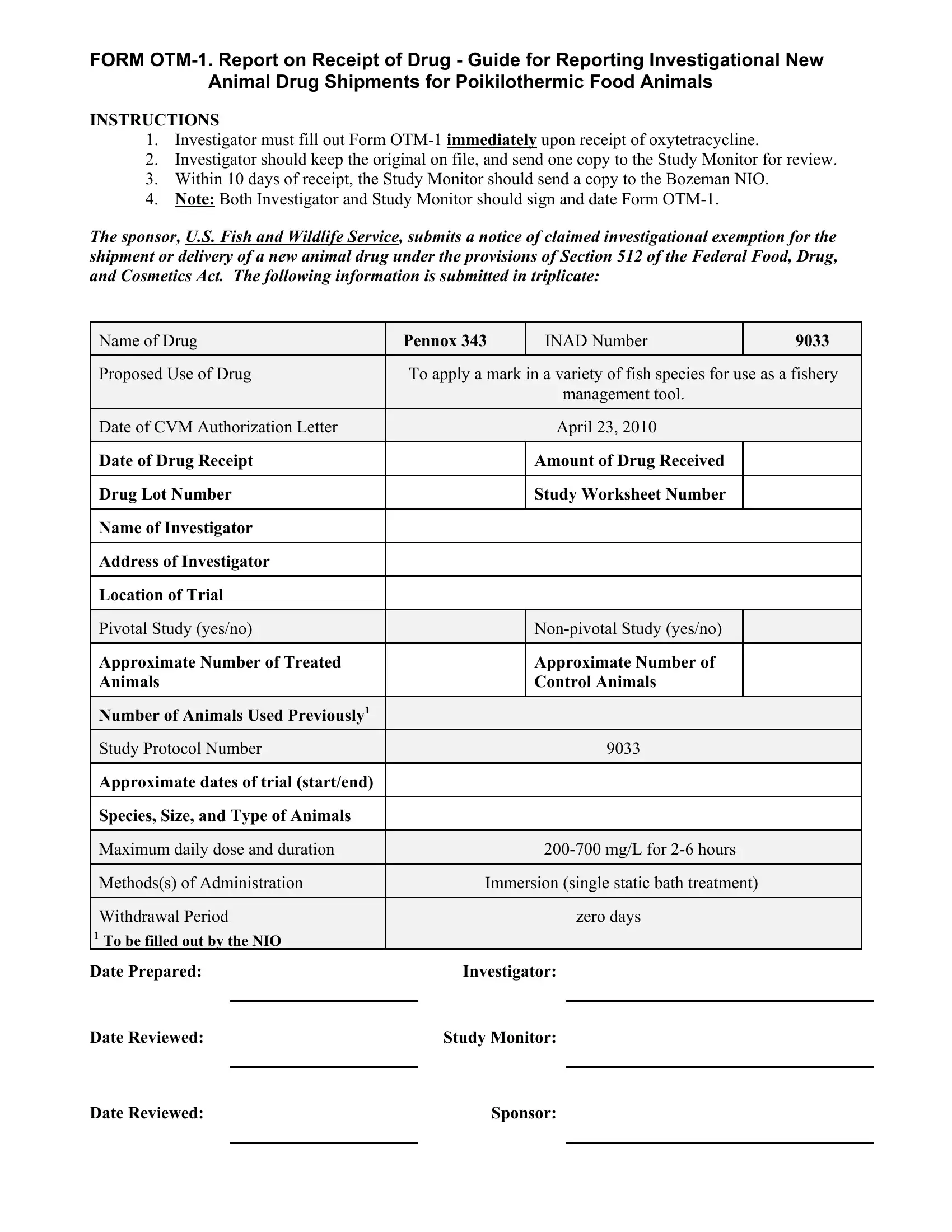
In case you desire to fill out Form Otm 1, you don't have to install any kind of applications - simply give a try to our PDF tool. FormsPal expert team is constantly endeavoring to improve the editor and enable it to be even easier for people with its handy features. Enjoy an ever-evolving experience today! With a few simple steps, it is possible to begin your PDF editing:
Step 1: Click on the "Get Form" button above. It'll open our pdf tool so that you could begin completing your form.
Step 2: With our handy PDF tool, it is possible to accomplish more than merely fill in blanks. Express yourself and make your documents appear professional with customized textual content added, or fine-tune the original input to perfection - all comes along with the capability to incorporate your personal images and sign the document off.
As for the fields of this particular form, here's what you should do:
1. When filling out the Form Otm 1, make sure to complete all important blank fields within the relevant area. It will help to expedite the process, which allows your information to be handled fast and appropriately.
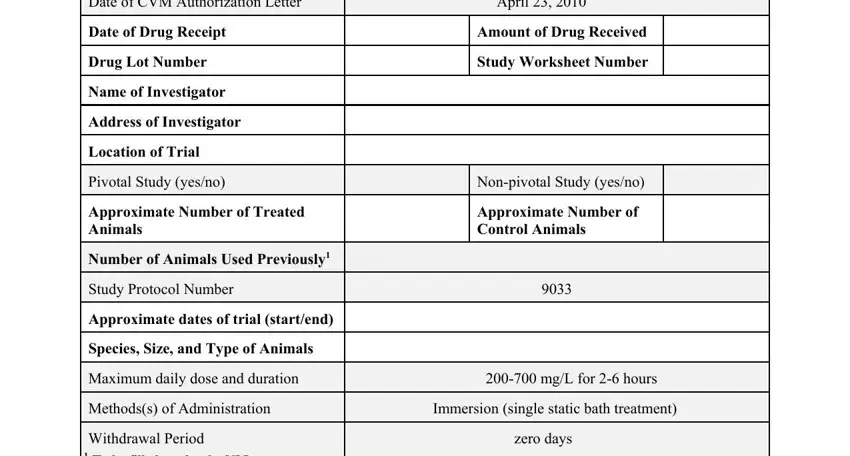
2. Just after performing this step, go to the subsequent stage and enter the necessary particulars in these blank fields - Date of CVM Authorization Letter, Investigator, Date Reviewed, Study Monitor, Date Reviewed, and Sponsor.
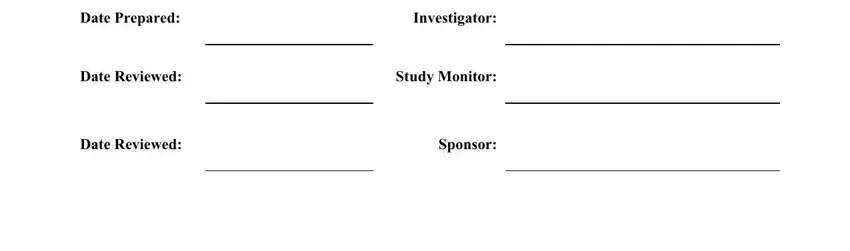
It is easy to make an error while completing your Investigator, for that reason make sure that you look again before you decide to send it in.
Step 3: Before moving on, ensure that blank fields have been filled in right. Once you think it is all fine, press “Done." After getting afree trial account here, you'll be able to download Form Otm 1 or send it via email right off. The PDF form will also be available through your personal cabinet with your edits. FormsPal is devoted to the personal privacy of our users; we make sure all personal information processed by our system remains confidential.