Form Approved Through 06/30/2012
OMB No. 09250001
DEPARTMENT OF HEALTH AND HUMAN SERVICES
Procedure for Submission of
Final Invention Statement and Certification (For Grant or Award)
Form HHS 568
A Final Invention Statement and Certification (Form HHS 568) shall be executed and submitted within 90 days following the expiration or termination of a grant or award. The Statement shall include all inventions which were conceived or first actually reduced to practice during the course of work under the grant or award, from the original effective date of support through the date of completion or termination. The Statement shall include any inventions reported previously for the grant or award as part of a noncompeting application. This reporting requirement is applicable to grants and awards by Department of Health and Human Services in support of research.
The Final Invention Statement and Certification does not in any way relieve the person responsible for the grant or award, or the institution, of the obligation to assure that all inventions are promptly and fully reported directly to the National Institutes of Health, as required by terms of the grant or award. Information regarding the reporting of inventions, including the reporting form to be followed, may be obtained from the Office of Policy for Extramural Research Administration, Division of Extramural Inventions and Technology Resources, 6705 Rockledge Drive MSC 7980, Bethesda, Maryland 208927980, Telephone: (301) 4351986.
The original of the completed Final Invention Statement and Certification is to be returned to the awarding component that funded the grant or award. The entire grant or award number must appear in the designated box on the form. The period covered by the Final Invention Statement is the project period of the grant or award at a particular grantee institution. If no inventions were involved, insert the word “None” in the first block under item Title of Invention. Each Statement requires the signature of an institution official authorized to sign on behalf of the institution.
The PHS estimates that it will take from 5 to 10 minutes to complete this form. This includes time for reviewing the instructions, gathering needed information, and completing and reviewing the form. An agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a currently valid OMB control number. If you have comments regarding this burden estimate or any other aspects of this collection of information, including suggestions for reducing this burden, send comments to: NIH, Project Clearance Office, 6701 Rockledge Drive MSC 7730, Bethesda, MD 208927730, ATTN: PRA (09250001). Do not send this form to these
addresses; they are for comments only.
HHS 568 (Rev. 06/09) — Instructions
Department of Health and Human Services
Final Invention Statement and Certification
(For Grant or Award)
Form Approved Through 06/30/2012 OMB No. 09250001
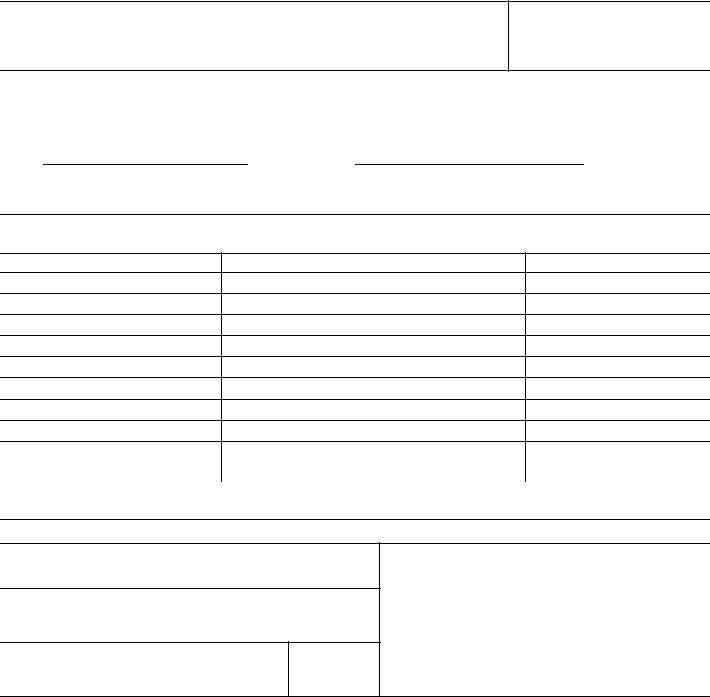
DHHS Grant or Award No.
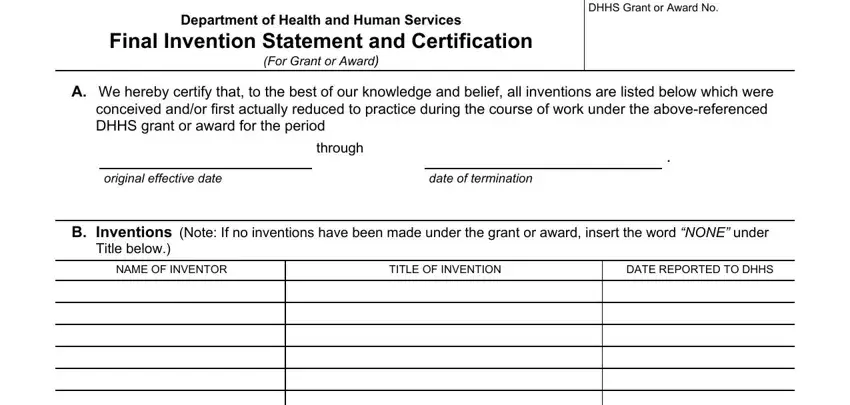
A.We hereby certify that, to the best of our knowledge and belief, all inventions are listed below which were conceived and/or first actually reduced to practice during the course of work under the abovereferenced DHHS grant or award for the period
through
.
original effective date |
date of termination |
B.Inventions (Note: If no inventions have been made under the grant or award, insert the word “NONE” under Title below.)
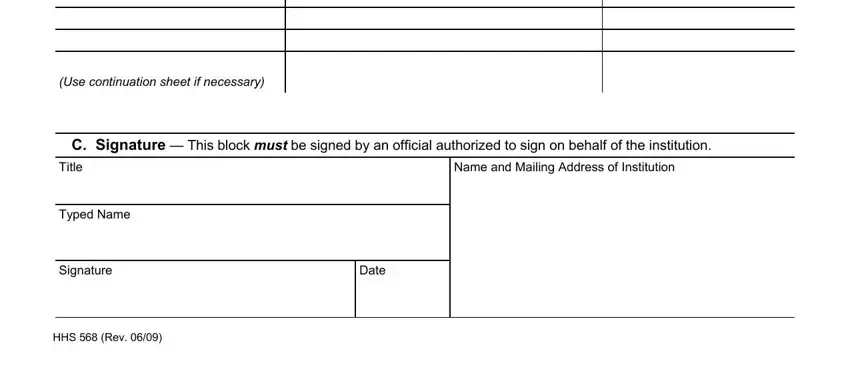
(Use continuation sheet if necessary)
C.Signature — This block MUST be signed by an official authorized to sign on behalf of the institution.
Name and Mailing Address of Institution
Privacy Act Statement
The PHS maintains application and grant records as part of a system of records as defined by the Privacy Act: 09250112, Grants and Cooperative Agreements: Research, Research Training, Fellowship, and Construction Applications and Related Awards.” The Privacy Act of 1974 (5 USC 522a) allows disclosures for “routine uses” and permissible disclosures.
Some routine uses may be:
1.To the cognizant audit agency for auditing.
2.To a Congressional office from a record of an individual in response to an inquiry from the Congressional office made at the request of that individual.
3.To qualified experts, not within the definition of DHHS employees as prescribed in DHHS regulations (45 CFR 5b.2) for opinions as part of the application review process.
4.To a Federal agency, in response to its request, in connection with the letting of a contract or the issuance of a license, grant, or other benefit by the requesting agency, to the extent that the record is relevant and necessary to the requesting agency’s decision on the matter;
5.To organizations in the private sector with whom PHS has contracted for the purpose of collating, analyzing, aggregating, or otherwise refining records in a system. Relevant records will be disclosed to such a contractor, who will be required to maintain Privacy Act safeguards with respect to such records.
6.To the sponsoring organization in connection with the review of an application or performance or administration under the terms and conditions of the award, or in connection with problems that might arise in performance or administration if an award is made.
7.To the Department of Justice, to a court or other tribunal, or to another party before such tribunal, when one of the following is a party to litigation or has any interest in such litigation, and the DHHS determines that the use of such records by the Department of Justice, the tribunal, or the other party is relevant and necessary to the litigation and would help in the effective representation of the governmental party.
a.the DHHS, or any component thereof;
b.any DHHS employee in his or her official capacity;
c.any DHHS employee in his or her individual capacity where the Department of Justice (or the DHHS, where it is authorized to do so) has agreed to represent the employee; or
d.the United States or any agency thereof; where the DHHS determines that the litigation is likely to affect the DHHS or any of its components.
8.A record may also be disclosed for a research purpose, when the DHHS:
a.has determined that the use or disclosure does not violate legal or policy limitations under which the record was provided, collected, or obtained;
b.has determined that the research purpose (1) cannot be reasonably accomplished unless the record is provided in individually identifiable form, and (2) warrants the risk to the privacy of the individual that additional exposure of the record might bring;
c.has secured a written statement attesting to the recipient’s understanding of; and willingness to abide by, these provisions; and
d.has required the recipient to:
(1)establish reasonable administrative, technical, and physical safeguards to prevent unauthorized use or disclosure of the record;
(2)destroy the information that identifies the individual at the earliest time at which removal or destruction can be accomplished consistent with the purpose of the research project, unless the recipient has presented adequate justification of a research or health nature for retaining such information; and
(3)make no further use or disclosure of the record, except (a) in emergency circumstances affecting the health or safety of any individual, (b) for use in another research project, under these same conditions, and with written authorization of the DHHS, (c) for disclosure to a properly identified person for the purpose of an audit related to the research project, if information that would enable research subjects to be identified is removed or destroyed at the earliest opportunity consistent with the purpose of the audit, or (d) when required by law.
The Privacy Act also authorizes discretionary disclosures where determined appropriate by the PHS, including to law enforcement agencies, to the Congress acting within its legislative authority, to the Bureau of the Census, to the National Archives, to the General Accounting Office, pursuant to a court order, or as required to be disclosed by the Freedom of Information Act of 1974(5 USC 552) and the associated DHHS regulations (45 CFR Part 5).
HHS 568 (Rev. 06/09)—Privacy Act