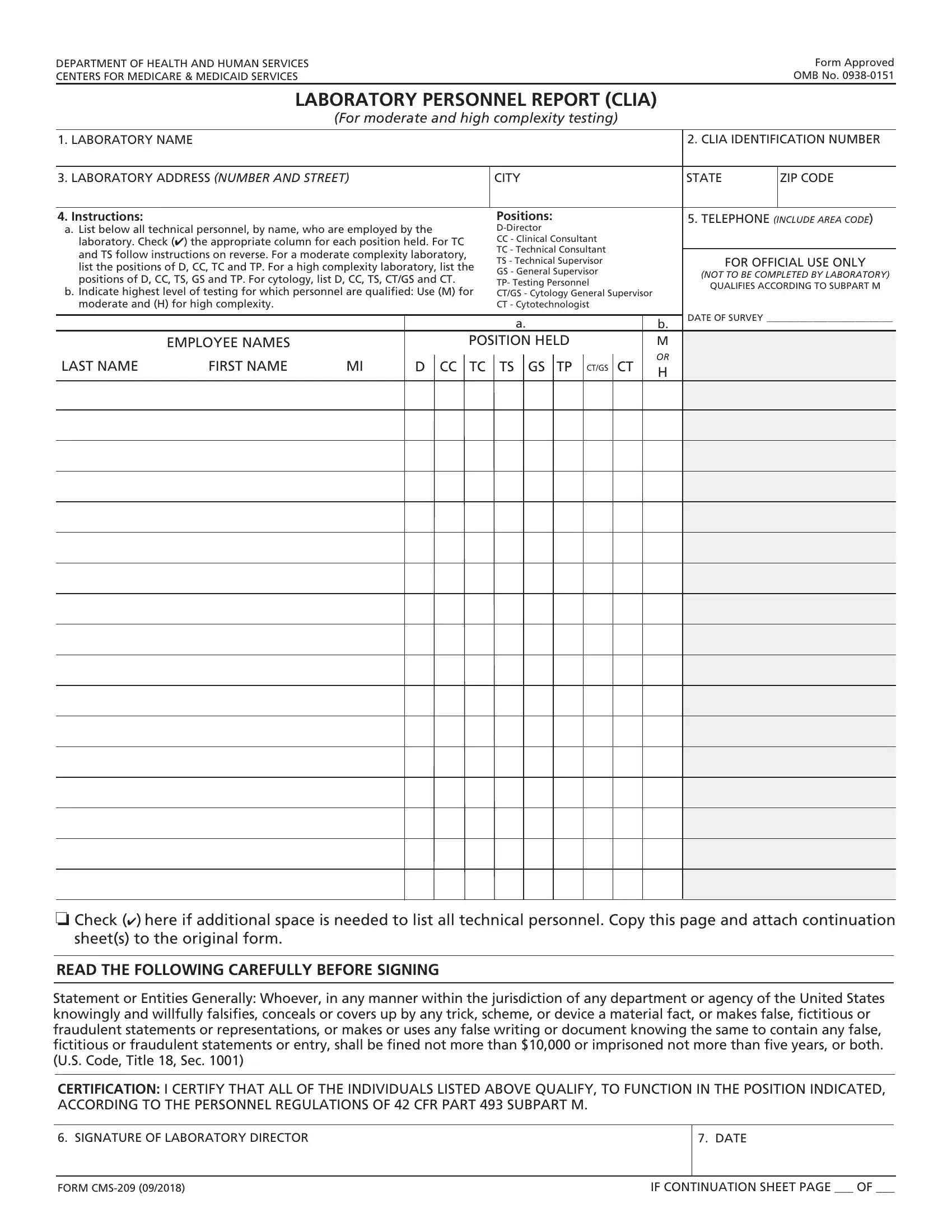
You'll be able to complete hcfa 209 instantly by using our online PDF editor. Our development team is continuously endeavoring to improve the editor and enable it to be much better for clients with its extensive features. Discover an ceaselessly revolutionary experience now - check out and find new opportunities as you go! All it requires is several basic steps:
Step 1: First of all, access the pdf editor by clicking the "Get Form Button" in the top section of this site.
Step 2: This tool lets you work with your PDF in a variety of ways. Transform it by adding your own text, correct original content, and add a signature - all when you need it!
Be mindful when filling in this form. Make certain every blank is filled out properly.
1. You should complete the hcfa 209 correctly, hence pay close attention while working with the parts that contain all of these blank fields:
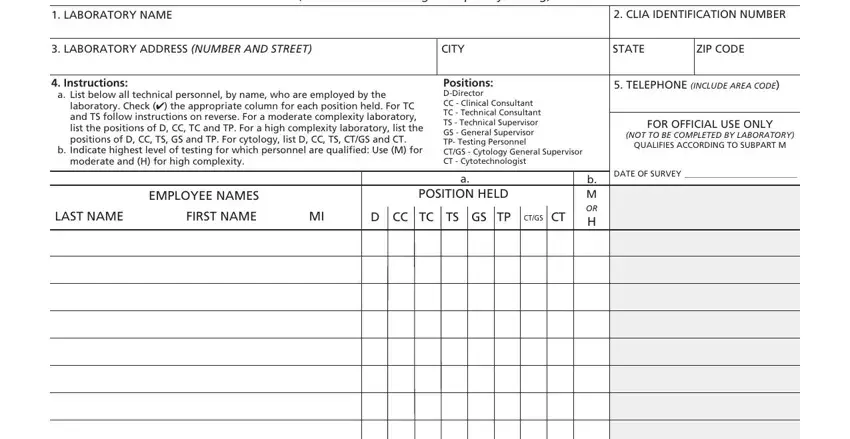
2. Right after the first selection of fields is filled out, proceed to enter the relevant details in these - o Check here if additional space, sheets to the original form, READ THE FOLLOWING CAREFULLY, and Statement or Entities Generally.
3. The next step is normally straightforward - fill out all the form fields in SIGNATURE OF LABORATORY DIRECTOR, DATE, FORM CMS, and IF CONTINUATION SHEET PAGE OF to complete this part.

Always be really careful when filling in IF CONTINUATION SHEET PAGE OF and DATE, because this is the part in which most users make mistakes.
Step 3: Confirm that your information is correct and press "Done" to conclude the process. Try a free trial plan at FormsPal and obtain direct access to hcfa 209 - which you'll be able to then begin using as you wish in your FormsPal cabinet. FormsPal offers protected form completion with no data record-keeping or any type of sharing. Feel at ease knowing that your data is secure here!