You could fill out application for certificate of compliance without difficulty with our PDFinity® editor. To retain our tool on the leading edge of practicality, we strive to put into operation user-driven capabilities and improvements on a regular basis. We're routinely grateful for any feedback - join us in revolutionizing PDF editing. To start your journey, consider these simple steps:
Step 1: First, access the pdf tool by clicking the "Get Form Button" above on this page.
Step 2: Once you start the file editor, you will notice the document ready to be filled out. Other than filling in different blank fields, it's also possible to perform various other things with the PDF, including adding custom words, modifying the original textual content, inserting images, affixing your signature to the PDF, and more.
This PDF form will require particular details to be filled in, hence you need to take your time to enter what's required:
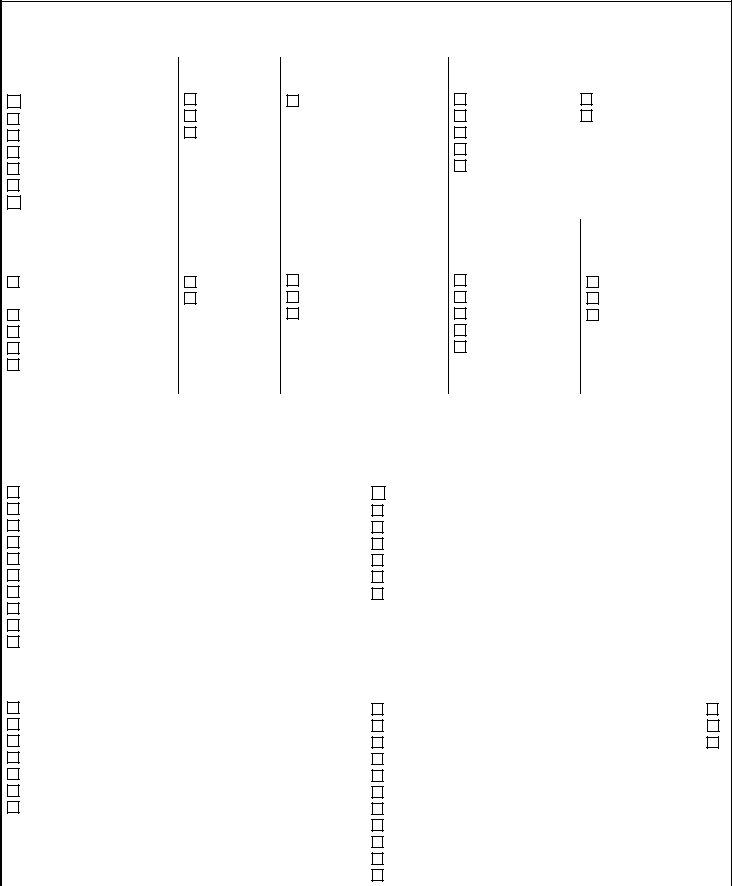
1. First, once filling in the application for certificate of compliance, start with the form section that includes the next fields:
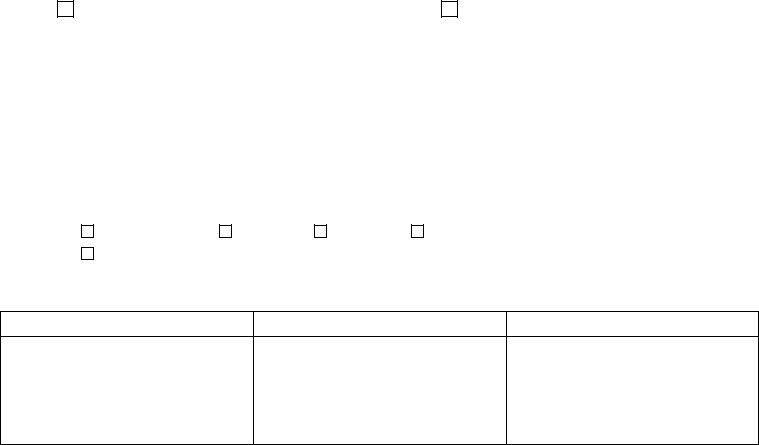
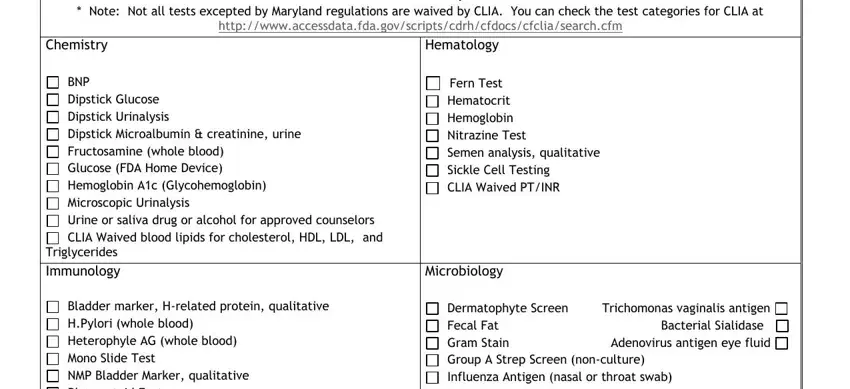
2. Soon after filling out the previous section, go on to the subsequent step and fill in the essential particulars in these blanks - Note Not all tests excepted by, V Schedule B Excepted Tests, Chemistry, BNP Dipstick Glucose Dipstick, Triglycerides, Immunology, Bladder marker Hrelated protein, Hematology, Fern Test Hematocrit Hemoglobin, Microbiology, and Dermatophyte Screen Trichomonas.
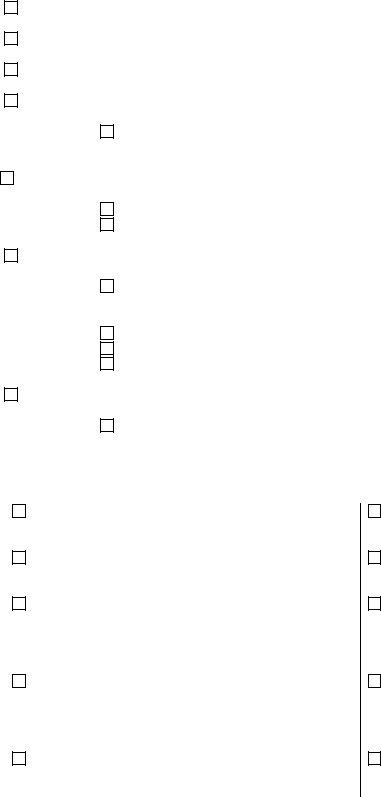
3. This 3rd step should be relatively easy, Chemistry Alkaline Phosphatase, Hematology APTT CBC Differential, and Pathology Dermatopathology Fine - all these form fields is required to be filled in here.

It is easy to make an error while filling in your Hematology APTT CBC Differential, therefore make sure that you look again prior to deciding to finalize the form.
Step 3: Before obtaining the next step, make certain that all blank fields are filled out right. When you believe it is all fine, click “Done." After registering a7-day free trial account with us, you'll be able to download application for certificate of compliance or send it through email right off. The file will also be readily available in your personal account menu with your adjustments. FormsPal guarantees safe form editor with no data record-keeping or any type of sharing. Rest assured that your data is safe here!