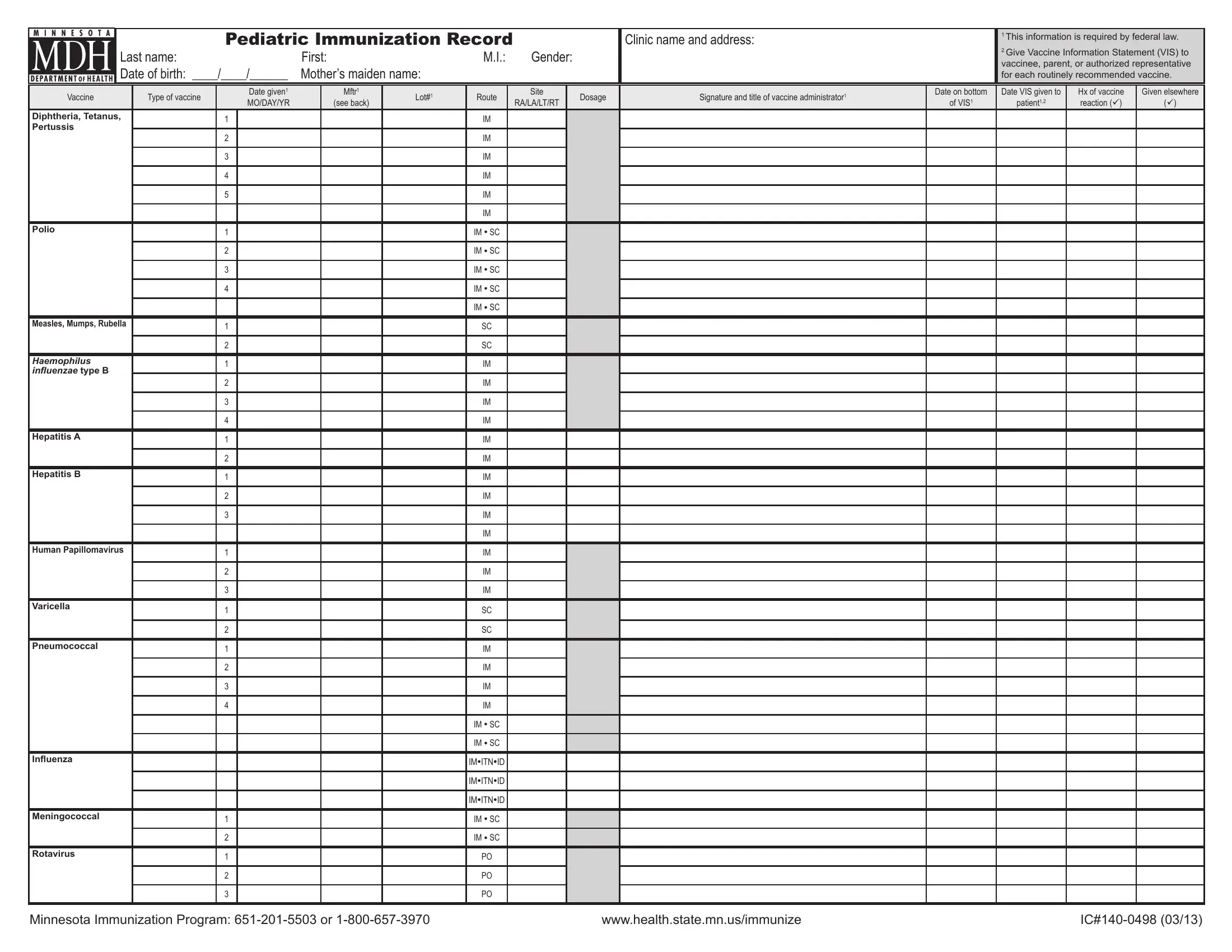
When working in the online PDF editor by FormsPal, you'll be able to fill out or edit IPV here. To make our tool better and simpler to use, we constantly work on new features, with our users' suggestions in mind. To get the ball rolling, consider these basic steps:
Step 1: Press the orange "Get Form" button above. It's going to open our pdf tool so you could begin filling out your form.
Step 2: This tool gives you the capability to customize your PDF in a range of ways. Transform it by writing your own text, correct what is originally in the document, and place in a signature - all close at hand!
This form will involve specific details; to guarantee accuracy and reliability, don't hesitate to take note of the suggestions further on:
1. When completing the IPV, be certain to incorporate all of the important blank fields within the associated area. This will help speed up the process, making it possible for your details to be handled without delay and appropriately.
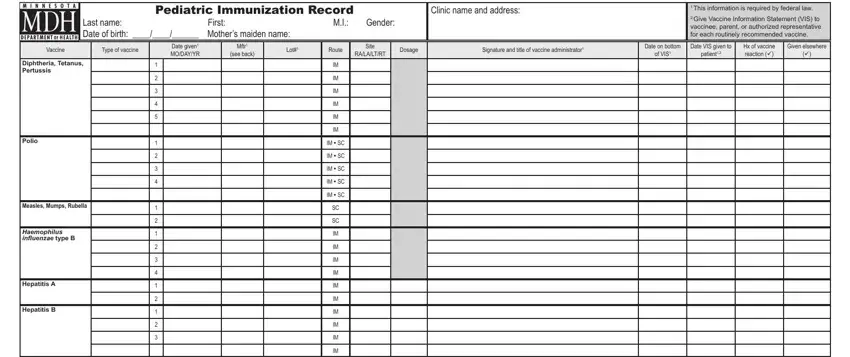
2. Once the last section is completed, go on to type in the applicable details in these - Human Papillomavirus, Varicella, Pneumococcal, Inluenza, Meningococcal, Rotavirus, IM SC, IM SC, IMITNID, IMITNID, IMITNID, IM SC, IM SC, Minnesota Immunization Program or, and wwwhealthstatemnusimmunize.
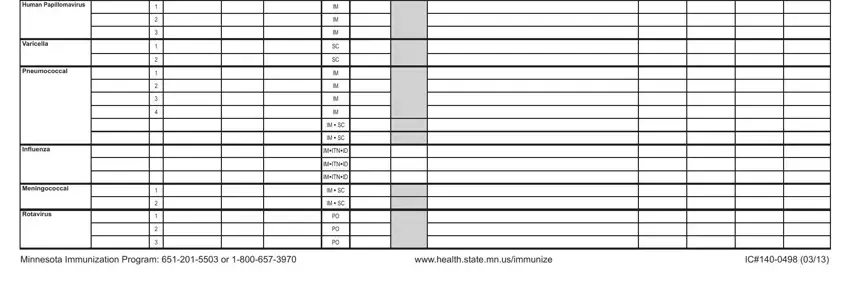
People who use this PDF generally make errors when completing Varicella in this section. You need to review what you enter here.
Step 3: Prior to submitting the file, it's a good idea to ensure that all blank fields were filled out as intended. The moment you’re satisfied with it, press “Done." Make a 7-day free trial subscription at FormsPal and get immediate access to IPV - accessible from your personal account page. FormsPal offers secure document editor with no data recording or distributing. Rest assured that your information is in good hands here!