ZZ113-120
RC Form 251-2 APPLICATION FOR A GENERAL LICENSEACKNOWLEDGEMENT (GLA)
INSTRUCTIONS: Complete this application in accordance with the instructions below. Retain a copy of the completed application for your files.
1.Legal business name and mailing address of applicant/licensee (Texas address only)
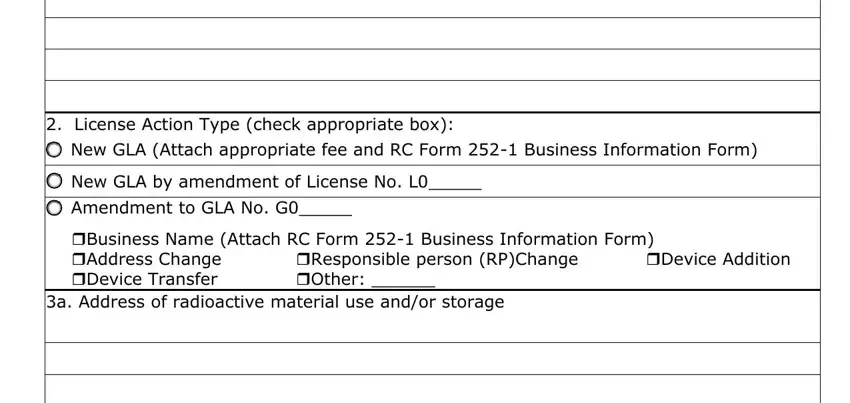
2.License Action Type (check appropriate box):
New GLA (Attach appropriate fee and RC Form 252-1 Business Information Form)
New GLA by amendment of License No. L0_____
Amendment to GLA No. G0_____
Business Name (Attach RC Form 252-1 Business Information Form)
Address Change |
Responsible person (RP)Change |
Device Addition |
Device Transfer |
Other: ______ |
|
3a. Address of radioactive material use and/or storage
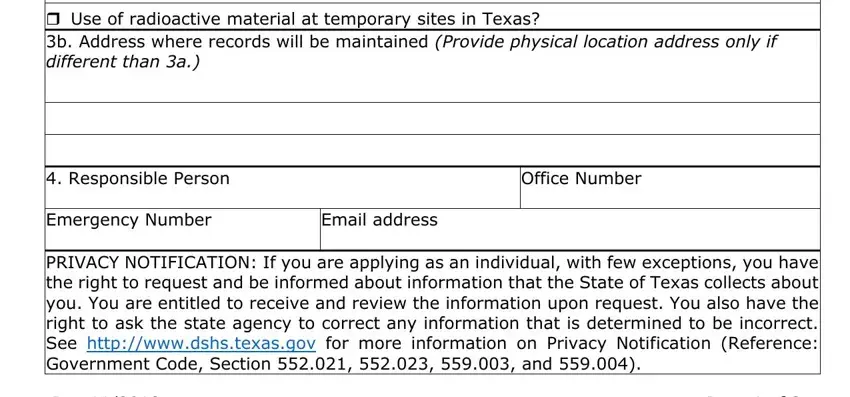
Use of radioactive material at temporary sites in Texas?
3b. Address where records will be maintained (Provide physical location address only if different than 3a.)
PRIVACY NOTIFICATION: If you are applying as an individual, with few exceptions, you have the right to request and be informed about information that the State of Texas collects about you. You are entitled to receive and review the information upon request. You also have the right to ask the state agency to correct any information that is determined to be incorrect. See http://www.dshs.texas.gov for more information on Privacy Notification (Reference: Government Code, Section 552.021, 552.023, 559.003, and 559.004).
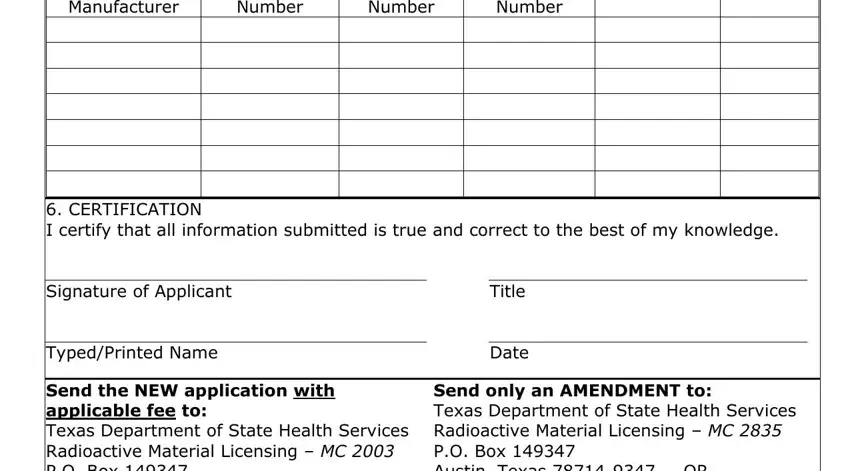
5.DEVICE DATA List for each device possessed and complete ALL fields (continue on a supplemental sheet if necessary)
6. CERTIFICATION
I certify that all information submitted is true and correct to the best of my knowledge.
Signature of Applicant
Typed/Printed Name
Send the NEW application with applicable fee to:
Texas Department of State Health Services
Radioactive Material Licensing – MC 2003
P.O. Box 149347
Austin, Texas 78714-9347
Title
Date
Send only an AMENDMENT to:
Texas Department of State Health Services
Radioactive Material Licensing – MC 2835
P.O. Box 149347
Austin, Texas 78714-9347 OR
RAMLicensing@dshs.texas.gov
INSTRUCTIONS FOR COMPLETING THE APPLICATION
Listed below are instructions for completing this application. Complete all applicable items. Use additional sheets if necessary.
For a NEW General License Acknowledgement (GLA), submit (1) this application form, (2)
RC Form 252-1 Business Information Form and (3) the appropriate 2-year application fee with the application. The review of the application will not begin until the appropriate application fee is paid.
For an AMENDMENT to a GLA, submit only this application form.
ITEM 1 - Indicate the legal business name and mailing address of the applicant or licensee in Texas. The applicant should be the corporation or other legal entity applying for the GLA. If the applicant is an individual, the individual should be acting in a private capacity and the use of the radioactive material should not be connected to the individual's employment with a corporation or other legal entity.
ITEM 2 - Check "New GLA" if the applicant does not have a GLA issued by the Agency. Check
“New GLA by amendment of License No. L0” and enter the license number if the applicant
requests the GLA on their radioactive material specific license. Check "Amendment of GLA No." and enter the GLA number if updating information previously submitted. When
amending the GLA, check the appropriate box that specifies the type of amendment you are requesting.
ITEM 3a - Specify the physical location of the radioactive material use and/or storage by street address, city, and zip code. If necessary, provide a descriptive address (e.g., five miles east of FM Road 14 on Highway 10) that will allow the agency to easily locate the facility. A post office box is not acceptable. If you intend to use a portable/mobile device throughout Texas, check the box "Use of radioactive material at temporary sites in Texas”. Radioactive material must not be used or stored in residential locations unless authorized by the agency.
ITEM 3b - Provide the physical address where records will be maintained if it is different than item 3a.
ITEM 4 - The Responsible Person (RP) is the individual responsible for ensuring compliance with the regulatory requirements. The RP maintains the GLA and associated records and is the primary contact with the Agency on matters pertaining to the GLA and use of the radioactive material. Enter the contact information of the RP.
ITEM 5 - Only report devices in accordance with 25 TAC §289.251(g)(1) containing at least
10 millicuries(mCi) of Cesium-137;
0.1 mCi of Strontium-90;
1 mCi of Cobalt-60;
0.1 mCi of Radium-226;
1 mCi of Americium-241 or any other transuranic (e.g. curium-244, californium-251);
The following information may be obtained by visual inspection of the permanently attached label located on the device. The application cannot be processed unless ALL the device information is provided.
Device Manufacturer - List the name of the manufacturer. Device Model Number - List the device model number Device Serial Number - List the device serial number.
Source Serial Number - List the serial number of the sealed sources.
Radionuclide & Activity - List the radionuclide, such as Cesium-137 (Cs-137) and include the activity for each sealed source. The activity should be expressed in curies (Ci), millicuries (mCi), or microcuries (µCi).
IF YOU CANNOT LOCATE ALL THE DEVICE DATA, CONTACT THE DEVICE MANUFACTURER OR DISTRIBUTOR FOR ASSISTANCE.
ITEM 6 - The application must be signed and dated by a person duly authorized to act on behalf of the applicant/licensee. The signature certifies the above information is true and correct as verified by a physical inventory and the information on the label of the device.
FEES
For a new GLA with 1 site, the fee is $1410.00.
For a new GLA with 1 site and a different records location, the fee is $1763.00.
There is no charge for an amendment to a current GLA registration.
For questions on fees, refer to Title 25 Texas Administrative Code (TAC) 25 TAC §289.204, contact us by phone at 512-231-5623 or email at RadiationFeesandRecords@dshs.texas.gov.