Our PDF editor works to make filling in forms stress-free. It is rather simple to edit the [FORMNAME] file. Keep up with these steps to be able to achieve this:
Step 1: Select the orange button "Get Form Here" on this page.
Step 2: You can now edit the printable temperature log. Our multifunctional toolbar helps you include, remove, adapt, and highlight content as well as undertake other sorts of commands.
For every single area, add the data requested by the system.
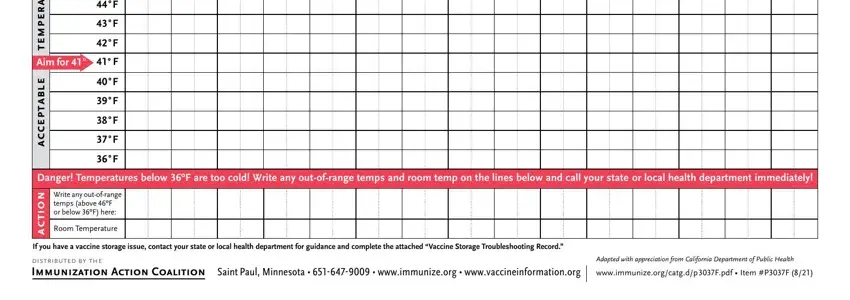
Note the details in s e r u t a r e p m e t, Aim for º, e l b a t p e c c a, F F F F F F F F F F F, Danger Temperatures below ºF are, n o, i t c a, Write any outofrange temps above, Room Temperature, If you have a vaccine storage, distributed by the Immunization, Adapted with appreciation from, and wwwimmunizeorgcatgdpFpdf Item PF.
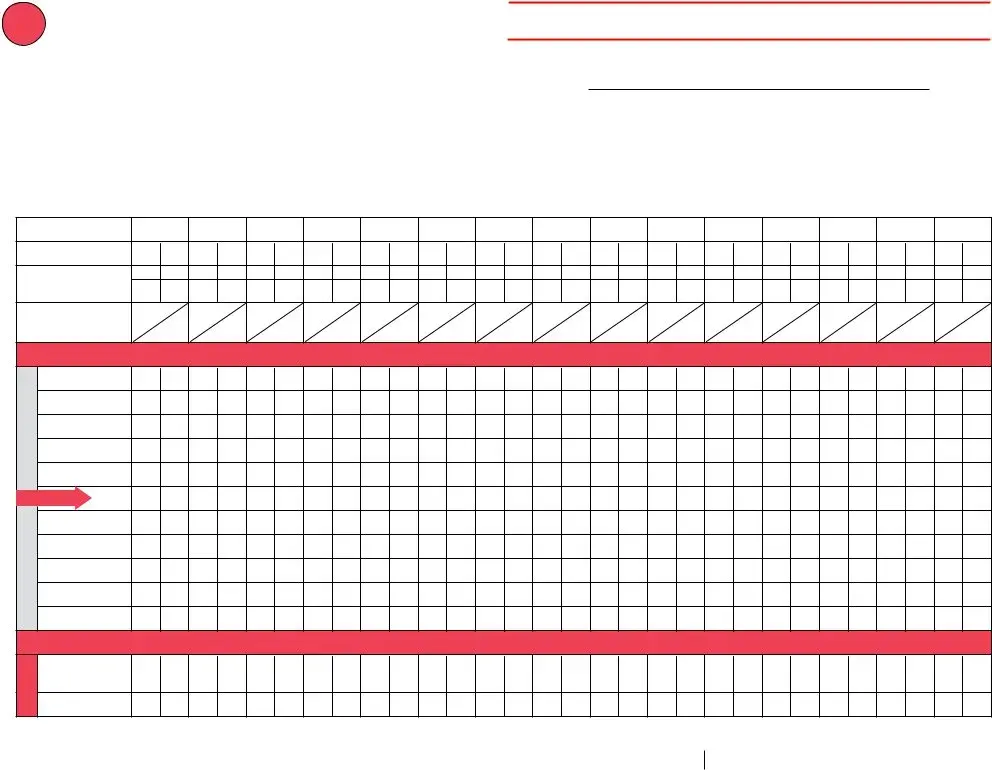
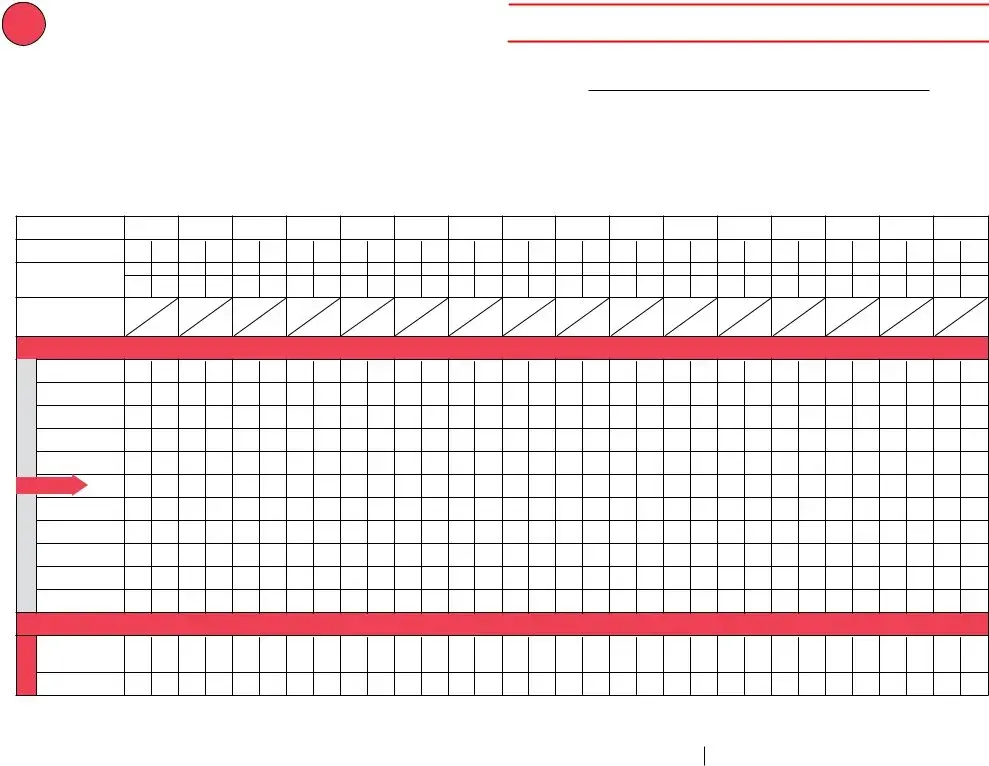
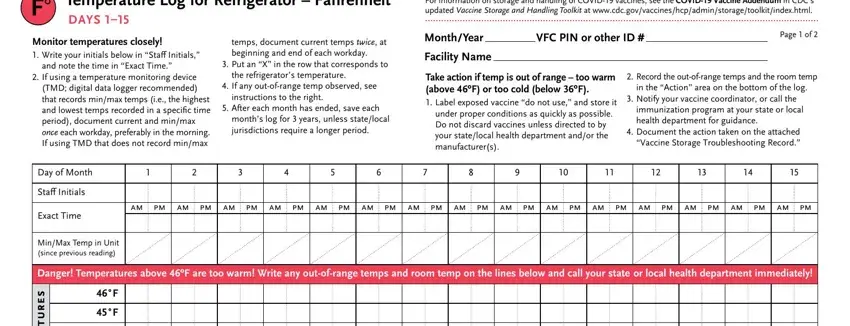
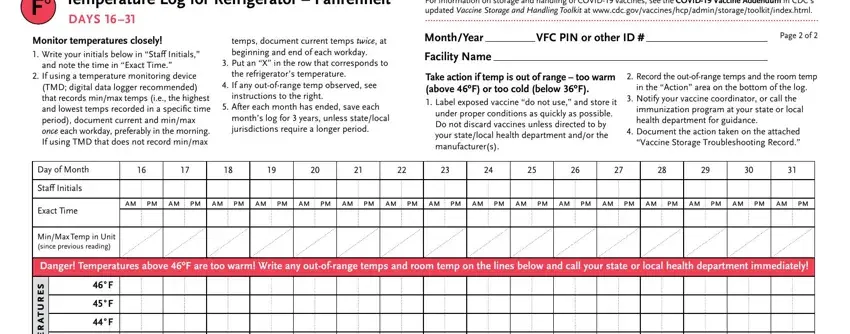
The application will require you to provide specific valuable information to automatically fill in the area Temperature Log for Refrigerator, Monitor temperatures closely, and note the time in Exact Time, If using a temperature monitoring, temps document current temps twice, Put an X in the row that, the refrigerators temperature, If any outofrange temp observed, instructions to the right, After each month has ended save, months log for years unless, For information on storage and, MonthYear VFC PIN or other ID, Page of, and Facility Name.
You should identify the rights and obligations of each party in field s e r u t a r e p m e t, Aim for º, e l b a t p e c c a, F F F F F F F F F F F, Danger Temperatures below ºF are, n o, i t c a, Write any outofrange temps above, Room Temperature, If you have a vaccine storage, distributed by the Immunization, Adapted with appreciation from, and wwwimmunizeorgcatgdpFpdf Item PF.

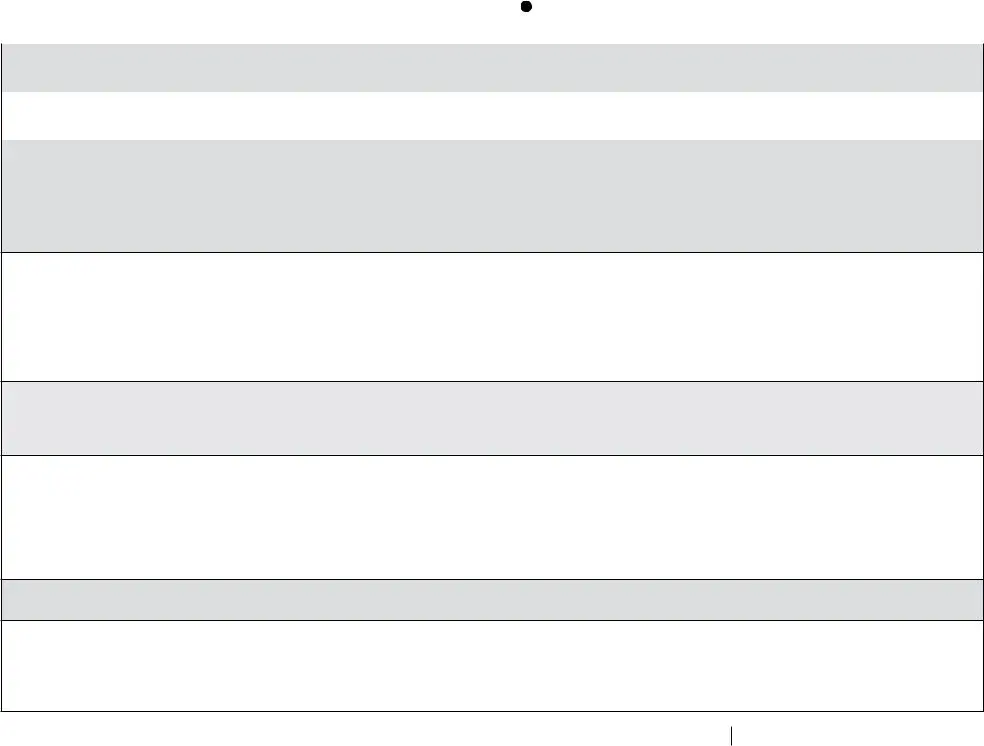
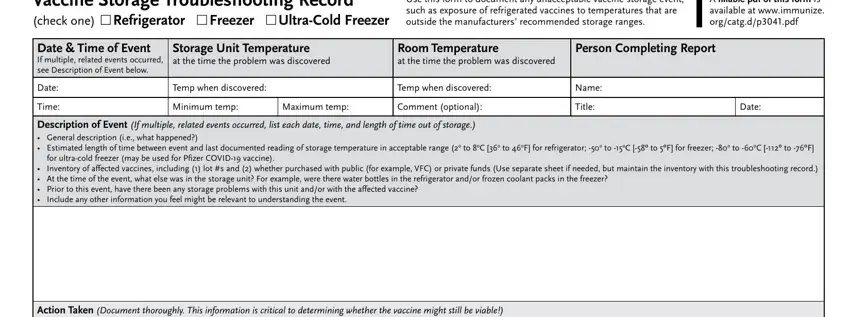
Review the areas Vaccine Storage Troubleshooting, Use this form to document any, A fillable pdf of this form is, Date Time of Event If multiple, Storage Unit Temperature at the, Room Temperature at the time the, Person Completing Report, Date, Time, Temp when discovered, Temp when discovered, Minimum temp, Maximum temp, Comment optional, and Name and next fill them out.
Step 3: Hit the Done button to be certain that your finalized file is available to be transferred to every device you use or mailed to an email you specify.
Step 4: Ensure that you stay clear of future challenges by producing as much as a pair of copies of your document.