The whole process of completing the influenza vaccine consent form is very effortless. Our team ensured our PDF editor is not difficult to work with and helps fill in any sort of form within minutes. Listed below are some of the steps you will need to take:
Step 1: Press the orange button "Get Form Here" on this web page.
Step 2: Right now, it is possible to edit your influenza vaccine consent form. This multifunctional toolbar makes it possible to include, eliminate, modify, highlight, and perform many other commands to the text and fields within the document.
The following areas will frame the PDF document that you will be completing:

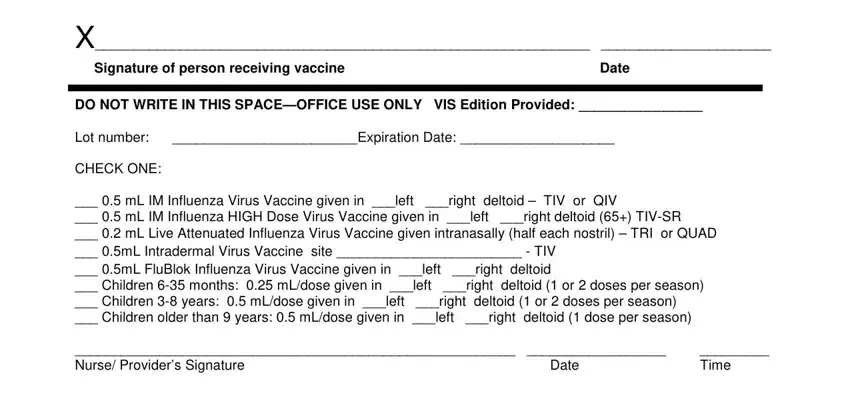
In the Signature of person receiving, Date, DO NOT WRITE IN THIS SPACEOFFICE, Lot number Expiration Date, CHECK ONE, mL IM Influenza Virus Vaccine, Nurse Providers Signature, Date, and Time area, put in writing your data.
Step 3: When you hit the Done button, the ready document is readily exportable to any type of of your devices. Alternatively, it is possible to deliver it via email.
Step 4: To avoid any hassles as time goes on, be sure to create a minimum of a couple of copies of the document.


