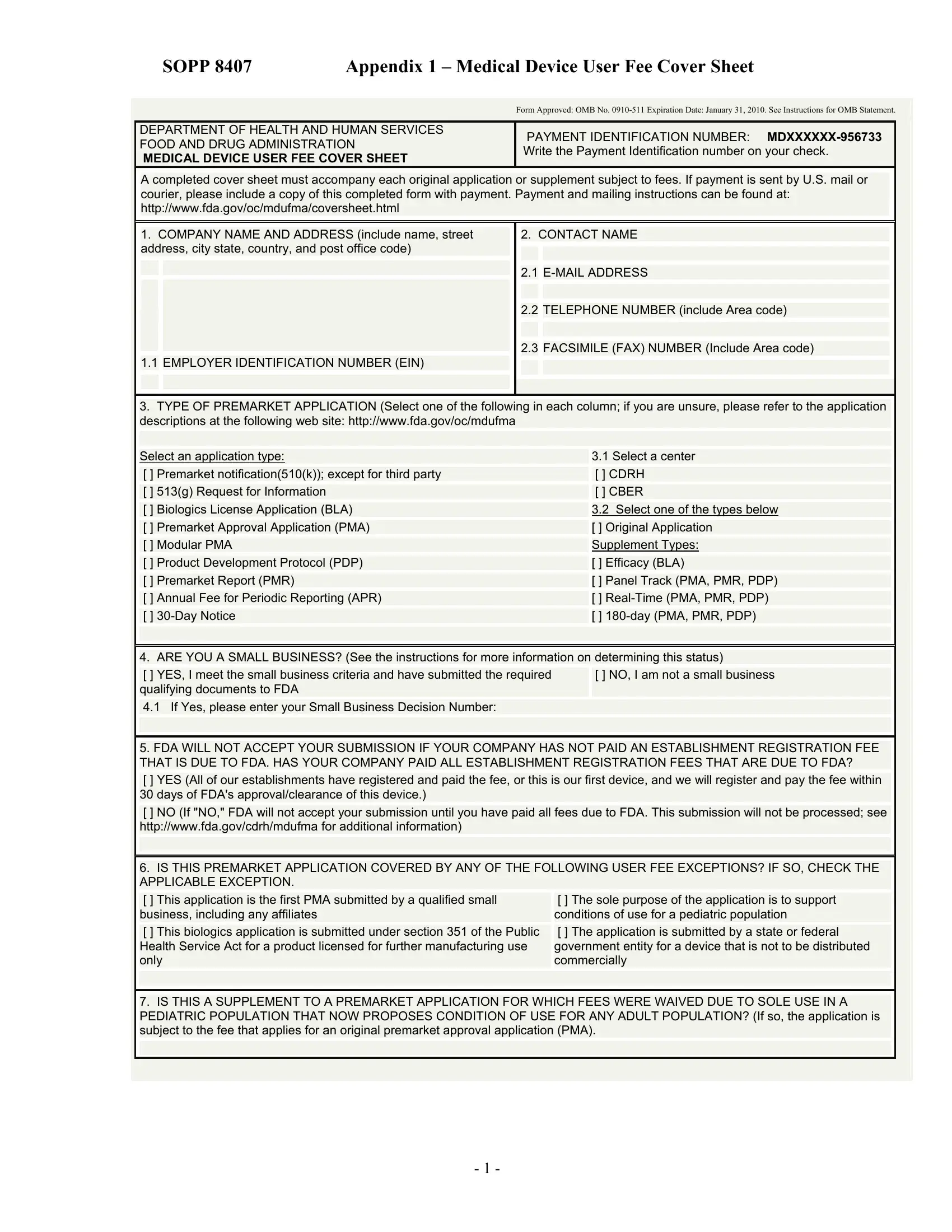
Any time you would like to fill out form 8407 appendix 1, it's not necessary to download and install any applications - simply try our online PDF editor. To make our editor better and less complicated to use, we consistently develop new features, with our users' feedback in mind. With a few easy steps, it is possible to begin your PDF journey:
Step 1: Firstly, access the tool by clicking the "Get Form Button" above on this webpage.
Step 2: With our advanced PDF editing tool, you can accomplish more than merely fill in forms. Express yourself and make your docs seem perfect with customized textual content put in, or fine-tune the file's original input to excellence - all accompanied by the capability to incorporate stunning photos and sign the file off.
To be able to fill out this PDF form, be certain to type in the required details in every single blank field:
1. Whenever filling out the form 8407 appendix 1, ensure to complete all of the important blank fields in its relevant part. This will help to expedite the process, which allows your information to be handled without delay and correctly.

2. The next part is to submit the following blank fields: Contact the FDA User Fees, If you are unsure whether or not, Contact Division of Small, Contact Office of Communication, Create MDUFMA User Fee Cover Sheet, and OMB No Form FDA.

It's simple to make errors while filling in your Contact the FDA User Fees, so make sure to look again prior to deciding to submit it.
Step 3: Right after you have looked again at the information provided, simply click "Done" to conclude your form at FormsPal. Make a 7-day free trial option at FormsPal and get immediate access to form 8407 appendix 1 - downloadable, emailable, and editable inside your FormsPal cabinet. FormsPal is invested in the privacy of our users; we always make sure that all information going through our tool is confidential.