This PDF editor makes it simple to manage the C6HsBr form. It's possible to prepare the form without delay through using these simple steps.
Step 1: Locate the button "Get Form Here" and select it.
Step 2: You can now edit the C6HsBr. Our multifunctional toolbar helps you include, remove, change, and highlight content material or perhaps carry out other sorts of commands.
If you want to prepare the C6HsBr PDF, provide the content for each of the segments:
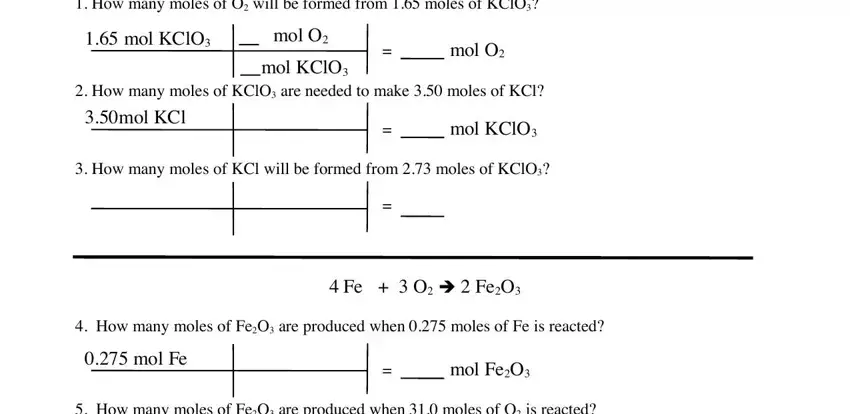
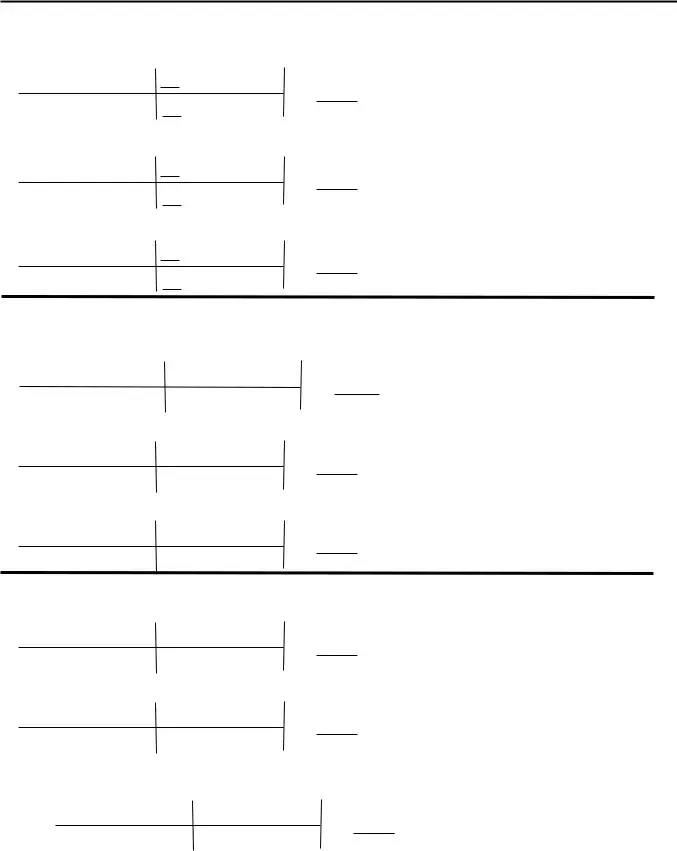
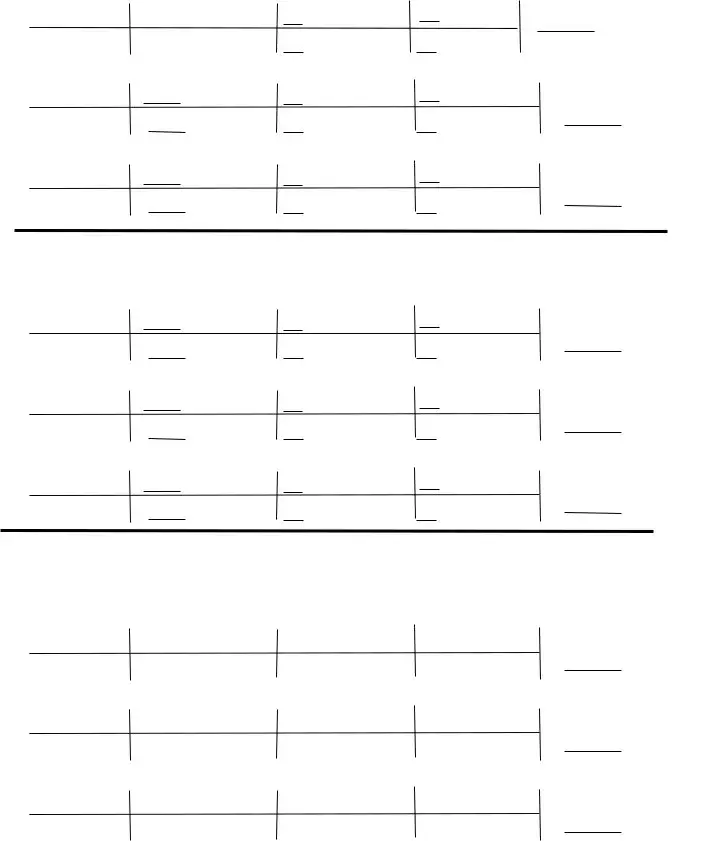
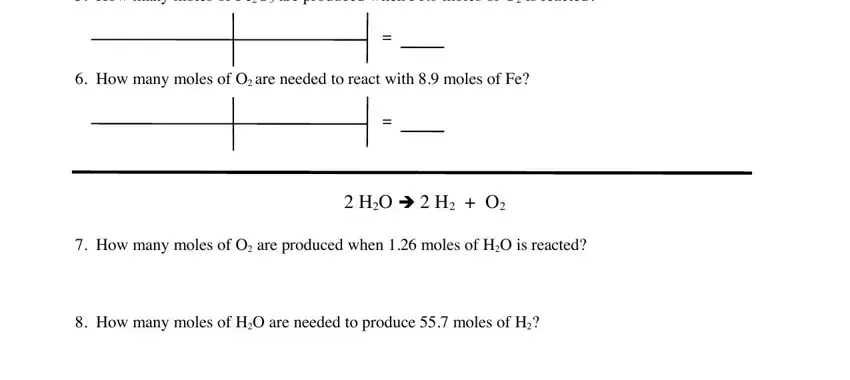
Complete the How many moles of FeO are, How many moles of O are needed to, HO è H O, How many moles of O are produced, and How many moles of HO are needed fields with any content that may be asked by the platform.
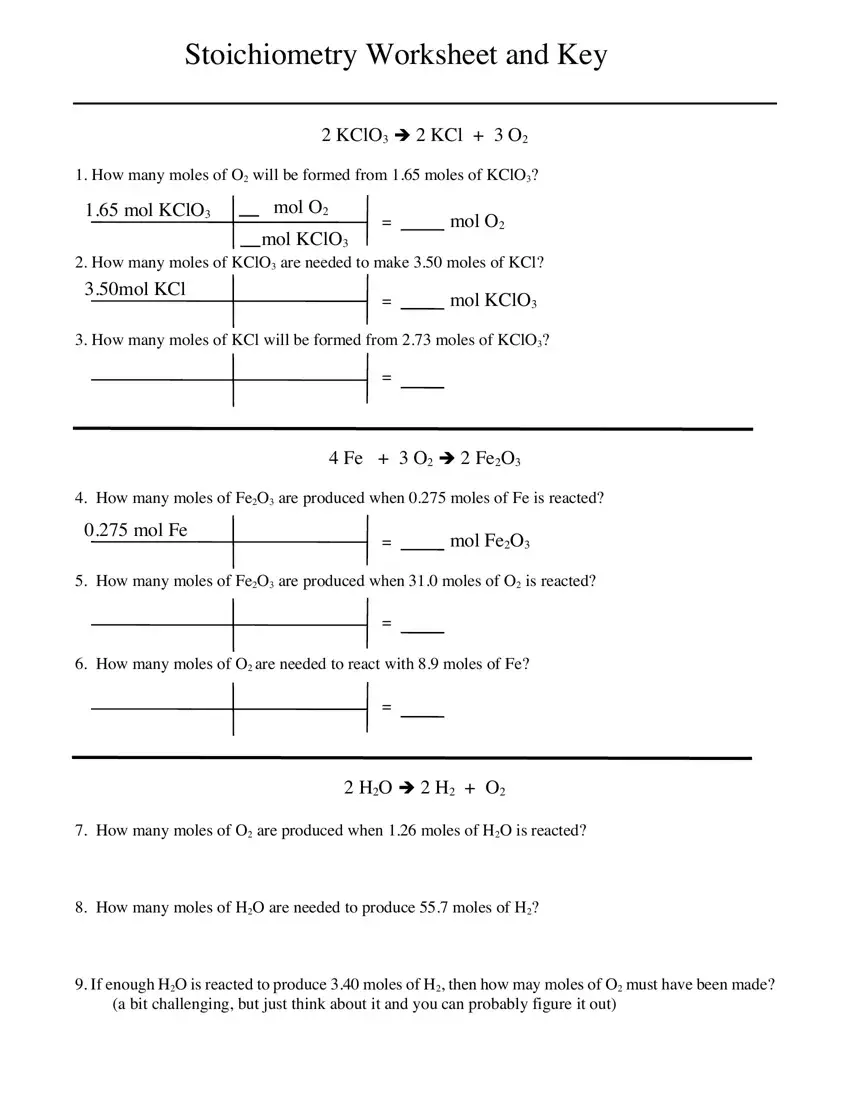
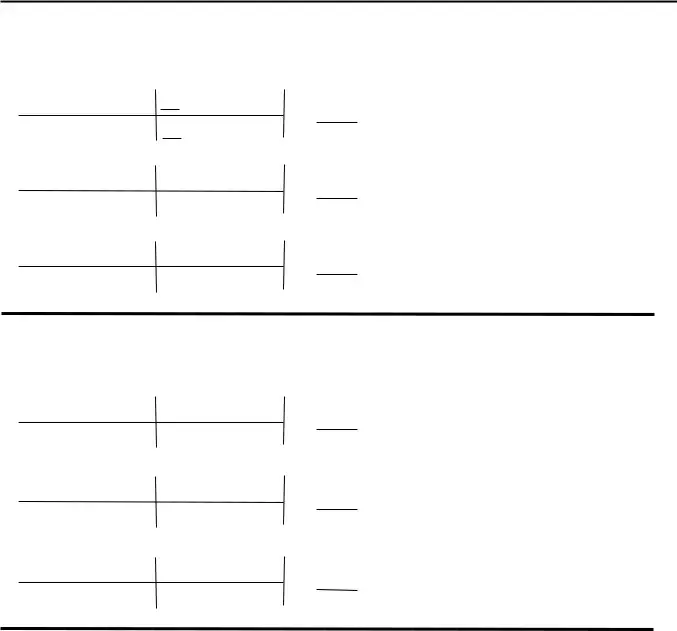
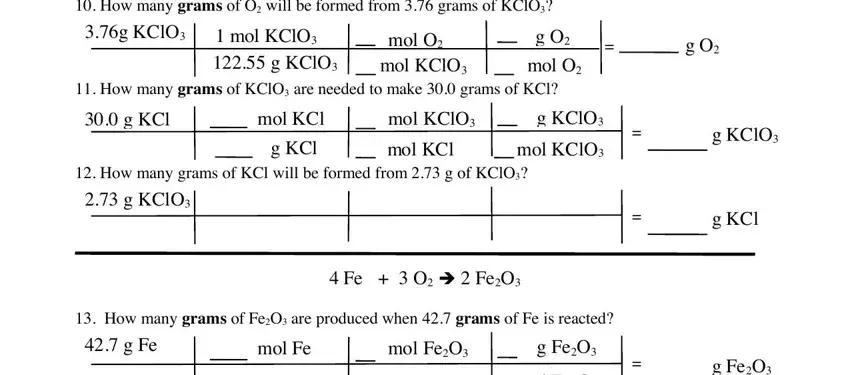
The system will ask you for data to automatically fill up the section How many grams of O will be, g KClO, mol KClO g KClO, mol O mol KClO, g O mol O, g O, How many grams of KClO are needed, g KCl, mol KCl, mol KClO, g KClO, How many grams of KCl will be, g KCl, mol KCl, and mol KClO.
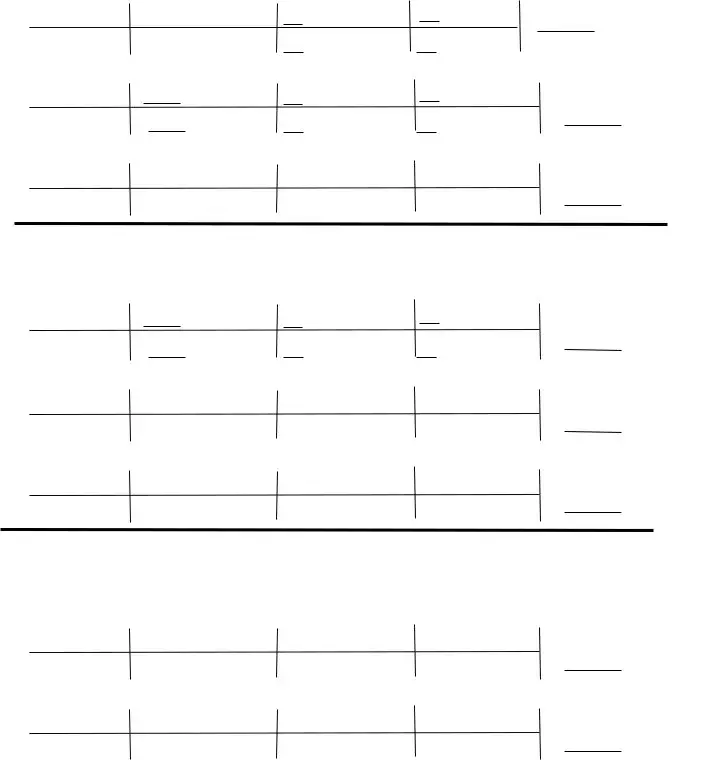
You will have to define the rights and obligations of every party in box How many grams of FeO are, g O, How many grams of O are needed to, g FeO, Some cars can use butane CH as fuel, CH O è CO HO, How many grams of CO are produced, g CH, How many grams of O are needed to, g CH, and g CO.
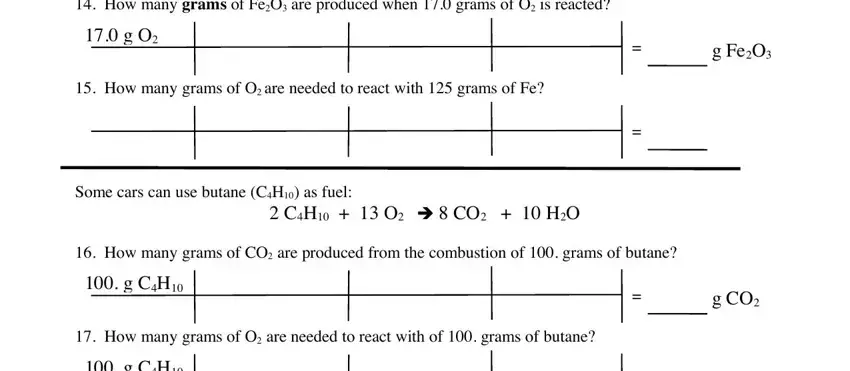
Finalize by looking at the following areas and completing them as required: g CH, How many grams of HO are produced, and g O.

Step 3: Select the Done button to be sure that your completed document may be exported to any type of electronic device you use or delivered to an email you specify.
Step 4: Make copies of the file - it will help you stay away from forthcoming issues. And don't get worried - we cannot distribute or look at your information.