Our top level web developers have worked collectively to make the PDF editor that you'll operate. The following software allows you to fill in student exploration chemical changes documents quickly and efficiently. This is all you need to do.
Step 1: Choose the "Get Form Now" button to begin the process.
Step 2: Now you may edit the student exploration chemical changes. You may use the multifunctional toolbar to insert, eliminate, and transform the content of the file.
Enter the information required by the application to complete the file.
Type in the necessary details in the area Check that the Visual display is, A How many hydrogen atoms are on, B How many oxygen atoms are on the, and Based on what you see is this.

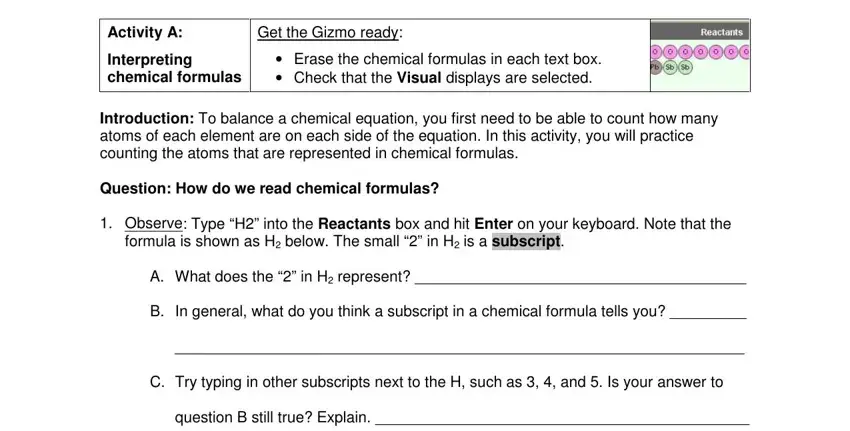
The software will request information to automatically fill out the box Activity A, Get the Gizmo ready, Interpreting chemical formulas, Erase the chemical formulas in, Introduction To balance a chemical, Question How do we read chemical, Observe Type H into the Reactants, formula is shown as H below The, A What does the in H represent, B In general what do you think a, C Try typing in other subscripts, and question B still true Explain.
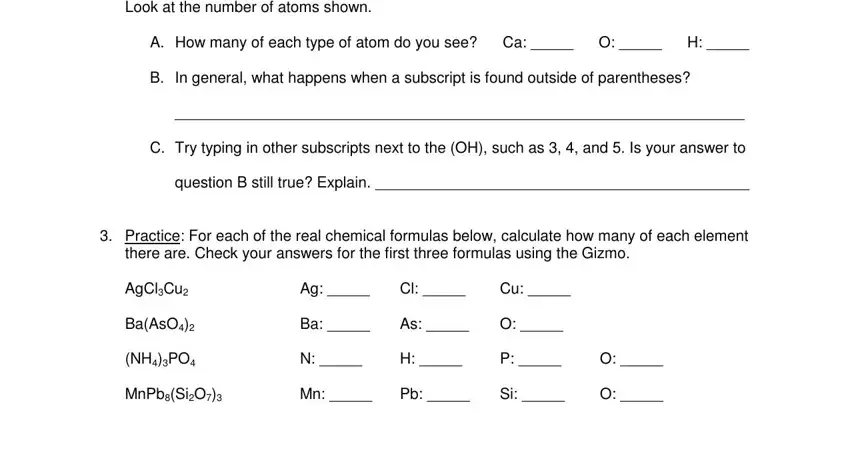
When it comes to space Look at the number of atoms shown, A How many of each type of atom do, B In general what happens when a, C Try typing in other subscripts, question B still true Explain, Practice For each of the real, there are Check your answers for, AgClCu, BaAsO, NHPO, and MnPbSiO, define the rights and obligations.
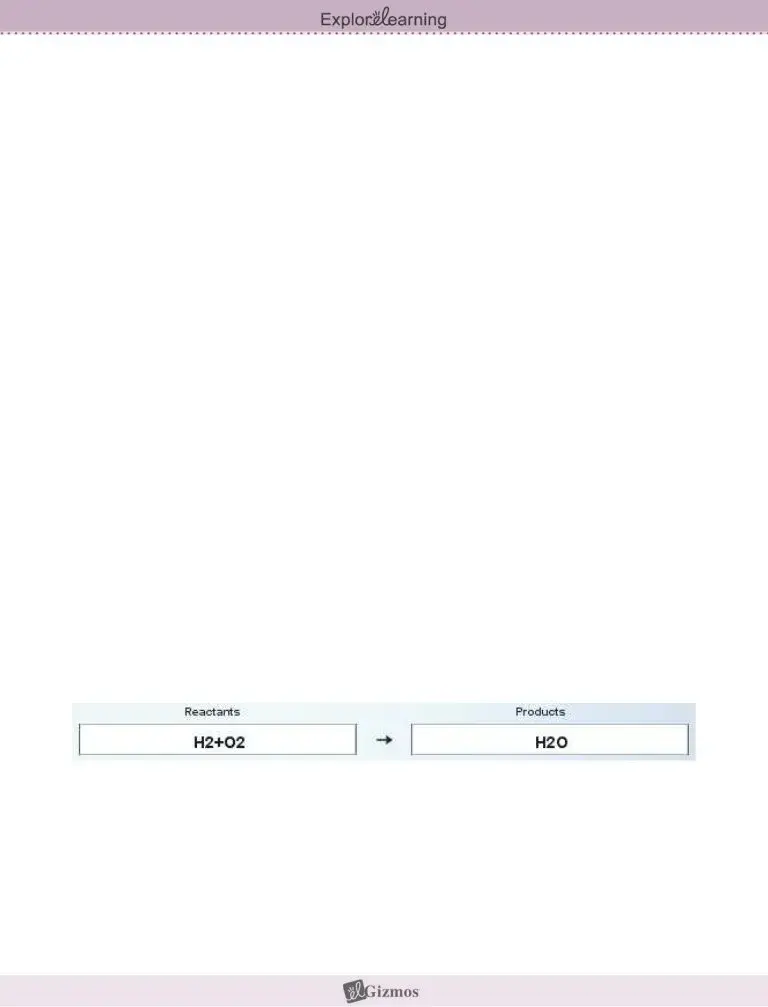
Review the sections Activity B, Balancing equations, Get the Gizmo ready, Erase the chemical formulas in, Introduction In a chemical, Goal Learn to balance any chemical, Observe To model how hydrogen and, Reactants box and HO into the, As the equation is written which, Explain, Balance To balance a chemical, and formulas of the substances and next fill them out.

Step 3: Select the button "Done". The PDF form may be exported. You can easily download it to your device or send it by email.
Step 4: In order to avoid any complications down the road, be sure to prepare up to a few duplicates of your file.