You are able to fill in vaccination records easily using our PDFinity® editor. In order to make our editor better and simpler to utilize, we consistently implement new features, with our users' suggestions in mind. In case you are seeking to begin, here's what it requires:
Step 1: Firstly, access the editor by pressing the "Get Form Button" at the top of this page.
Step 2: This tool offers you the ability to customize PDF forms in various ways. Transform it by writing personalized text, correct original content, and include a signature - all at your fingertips!
It is simple to complete the form using this helpful guide! Here is what you should do:
1. When filling out the vaccination records, ensure to incorporate all needed blank fields in their relevant form section. This will help to speed up the work, which allows your information to be handled promptly and appropriately.
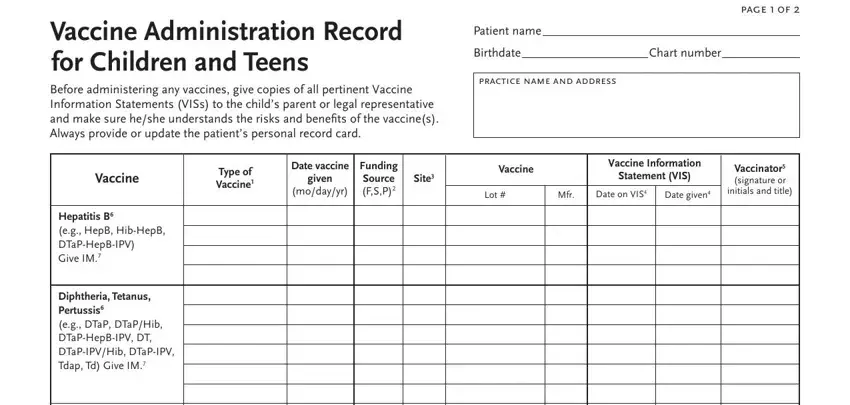
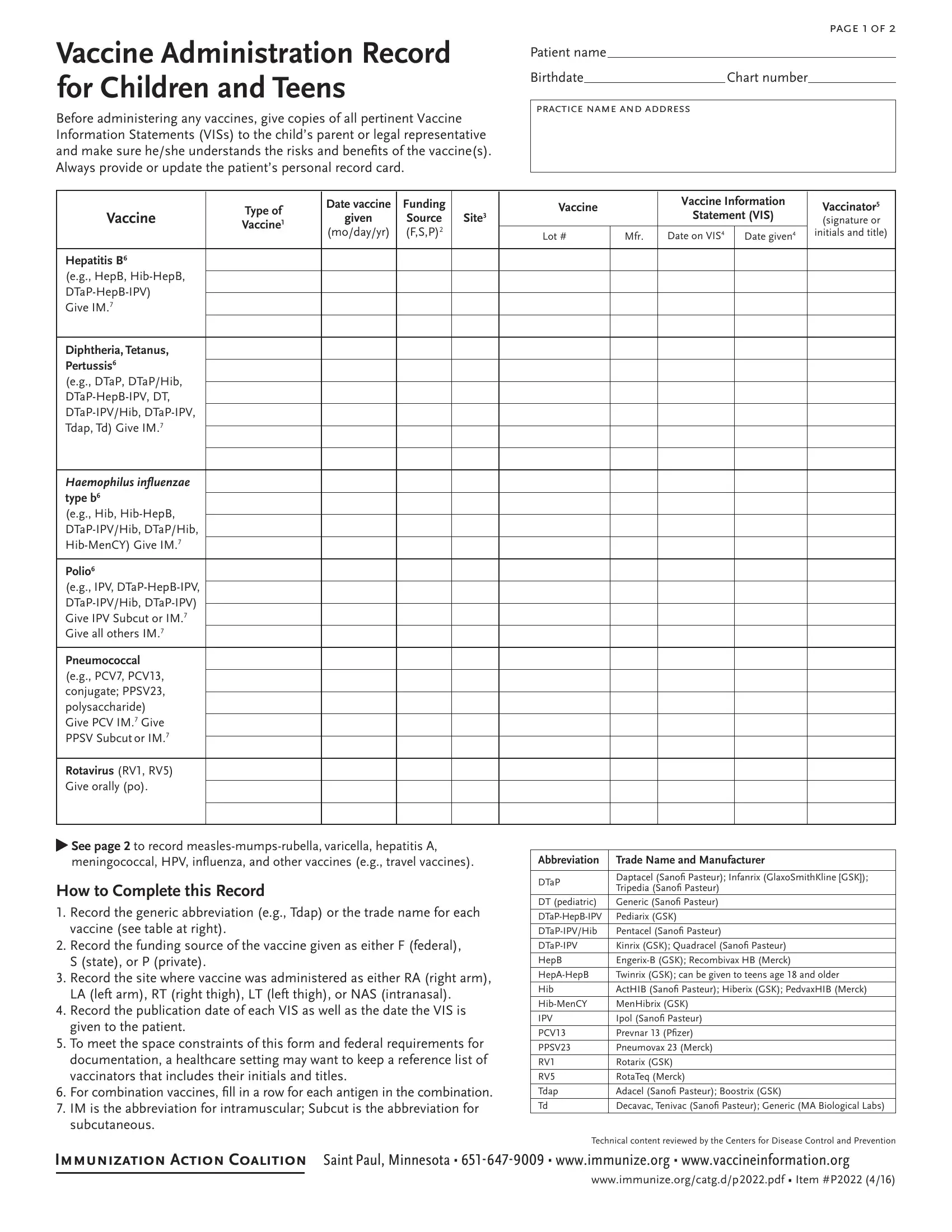
2. Right after completing the last section, go on to the subsequent part and fill out the essential details in these blanks - Haemophilus inluenzae type b eg, Polio eg IPV DTaPHepBIPV, Pneumococcal eg PCV PCV conjugate, Rotavirus RV RV Give orally po, See page to record, meningococcal HPV inluenza and, How to Complete this Record, vaccine see table at right, Record the funding source of the, S state or P private, Record the site where vaccine was, Abbreviation, DTaP, DT pediatric DTaPHepBIPV, and HepAHepB Hib HibMenCY IPV PCV PPSV.
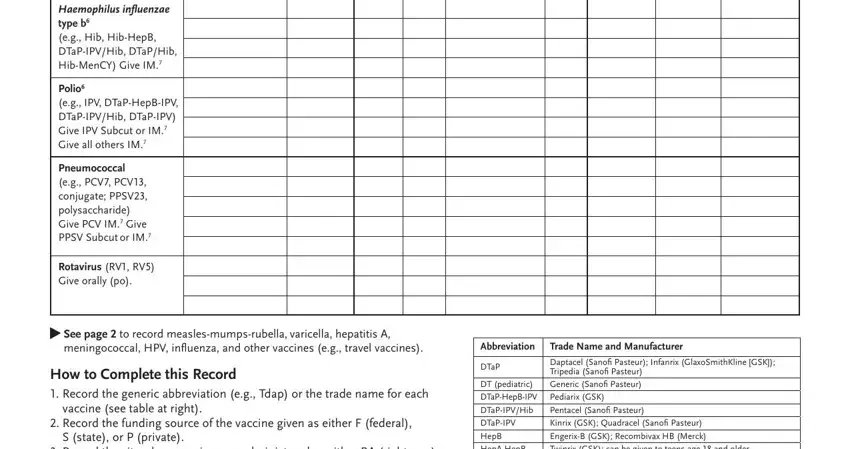
3. This next part will be focused on Immunization Action Coalition, and wwwimmunizeorgcatgdppdf Item P - complete all of these fields.

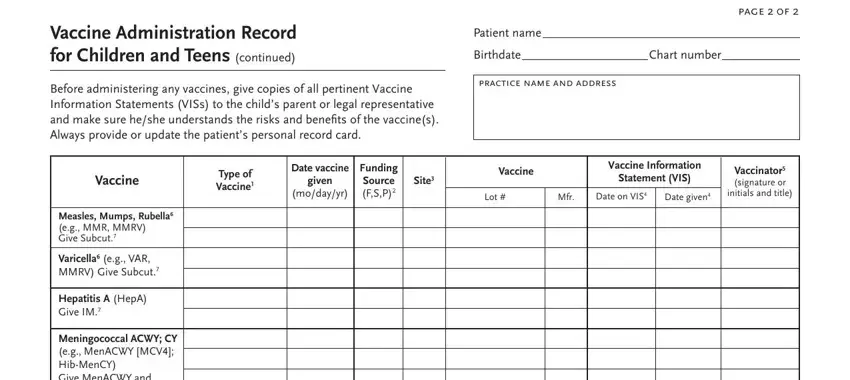
4. This next section requires some additional information. Ensure you complete all the necessary fields - Vaccine Administration Record for, Before administering any vaccines, page f, Patient name, Birthdate Chart number, practice name and address, Type of Vaccine, Date vaccine, given, modayyr, Funding Source FSP, Site, Vaccine, Vaccine Information, and Statement VIS - to proceed further in your process!
5. The document needs to be completed by dealing with this part. Here you will find an extensive set of form fields that need appropriate details to allow your document usage to be complete: Meningococcal ACWY CY eg MenACWY, Meningococcal B eg MenB Give MenB, Human papillomavirus eg HPV HPV, Inluenza eg IIV IIV ccIIV RIV LAIV, and Other.
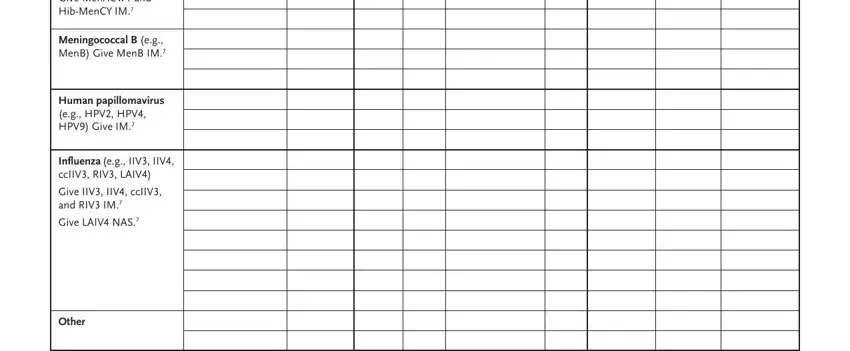
Be extremely attentive when filling in Meningococcal B eg MenB Give MenB and Inluenza eg IIV IIV ccIIV RIV LAIV, as this is where most users make some mistakes.
Step 3: Just after looking through the filled in blanks, hit "Done" and you're good to go! After starting afree trial account at FormsPal, you will be able to download vaccination records or email it promptly. The PDF will also be easily accessible in your personal account page with your edits. FormsPal ensures your information confidentiality with a protected method that in no way saves or distributes any private data involved. You can relax knowing your files are kept confidential each time you work with our service!