vaccine record card can be filled in online very easily. Just use FormsPal PDF editing tool to get it done promptly. Our development team is constantly working to improve the editor and enable it to be even easier for users with its many features. Make use of the latest progressive possibilities, and find a trove of new experiences! Here's what you'd want to do to get going:
Step 1: First of all, open the tool by pressing the "Get Form Button" above on this webpage.
Step 2: As soon as you access the PDF editor, you'll notice the form ready to be completed. Apart from filling in different fields, it's also possible to do other things with the file, that is putting on your own textual content, editing the original textual content, inserting illustrations or photos, signing the PDF, and much more.
This PDF will require specific details to be filled out, so be certain to take your time to fill in what's required:
1. While completing the vaccine record card, make sure to incorporate all of the needed fields in their corresponding area. This will help to facilitate the process, which allows your information to be processed fast and correctly.
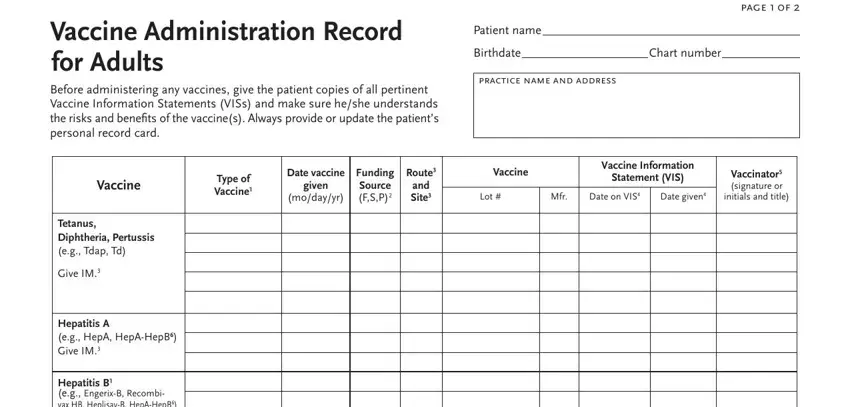
2. After completing the last part, go on to the next stage and complete the necessary details in all these blank fields - Hepatitis B eg EngerixB Recombi, Human papillomavirus HPV HPV HPV, Measles Mumps Rubella MMR Give, Varicella chickenpoxVAR Give Subcut, Meningococcal ACWY eg MenACWY MPSV, Meningococcal B eg MenB Give MenB, HPV HPV and MPSV vaccines are no, See page to record influenza, eg travel vaccines, How to Complete this Record With, Record the funding source of the, Abbreviation, Trade Name and Manufacturer, Tdap, and HepA.
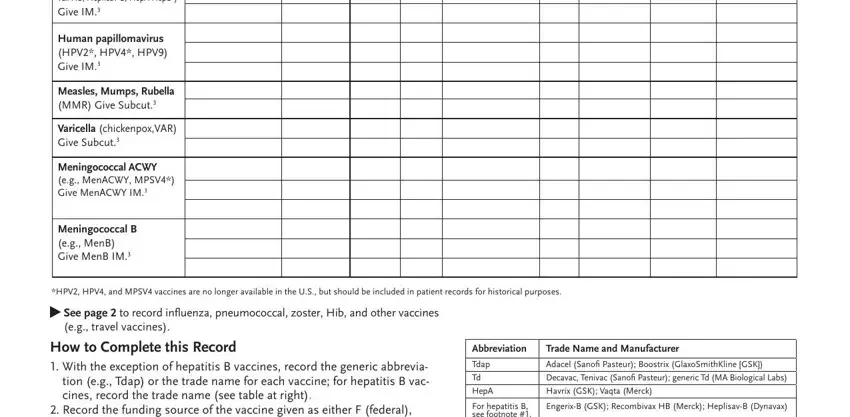
3. Throughout this part, review Vaccine Administration Record for, Before administering any vaccines, page f, Patient name, Birthdate, practice name and address, Chart number, Type of Vaccine, Date vaccine, given, modayyr, Funding Source FSP, Route and Site, Vaccine, and Vaccine Information. All of these will need to be taken care of with utmost focus on detail.
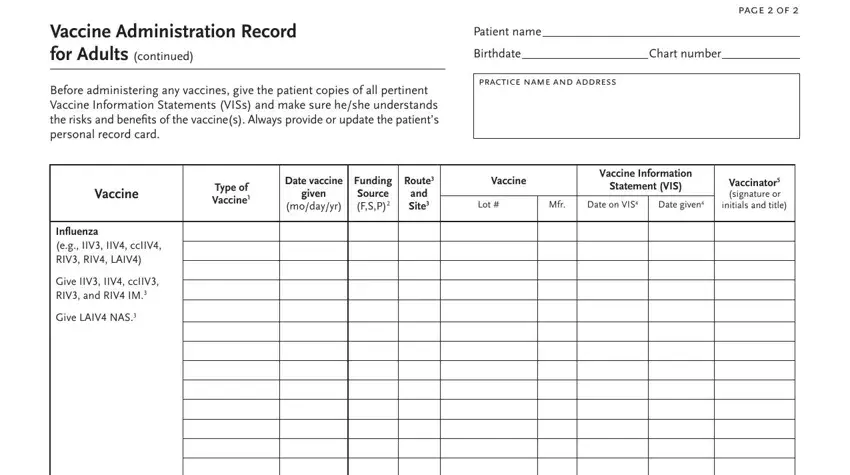
4. To go forward, this next stage will require typing in a few form blanks. Examples include Pneumococcal conjugate eg PCV Give, Pneumococcal polysac charide eg, Zoster shingles Give RZV IM Give, Hib Give IM, Other, See page to record TdapTd, MenACWY and MenB vaccines, How to Complete this Record, vaccine see table at right, Record the funding source of the, Abbreviation, Trade Name and Manufacturer, IIVIIV inactivated influenza, and Fluarix FluLaval GSK Afluria Fluad, which you'll find crucial to moving forward with this particular process.
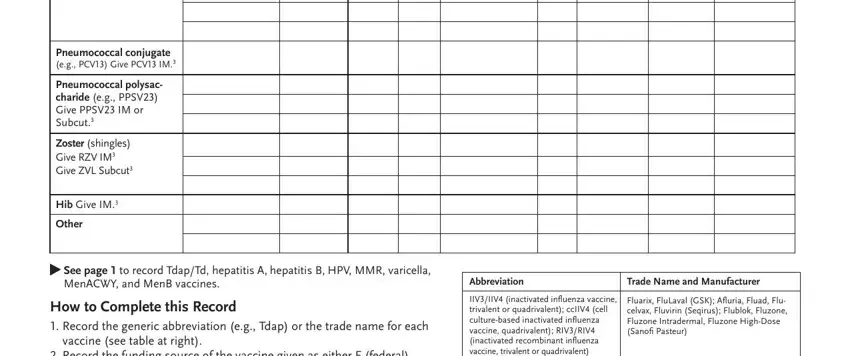
People who use this form generally get some points incorrect while filling in Record the funding source of the in this area. You need to revise whatever you enter here.
Step 3: Look through the details you have typed into the blank fields and click the "Done" button. Join FormsPal right now and easily get access to vaccine record card, ready for download. Each edit made is conveniently kept , meaning you can edit the file later if needed. FormsPal is focused on the privacy of our users; we make sure that all information going through our tool stays protected.