Managing the immunization form for school document is a breeze with our PDF editor. Follow these actions to prepare the document instantly.
Step 1: Step one is to click on the orange "Get Form Now" button.
Step 2: After you've accessed the editing page immunization form for school, you will be able to find each of the actions readily available for the document within the upper menu.
These parts will help make up your PDF document:
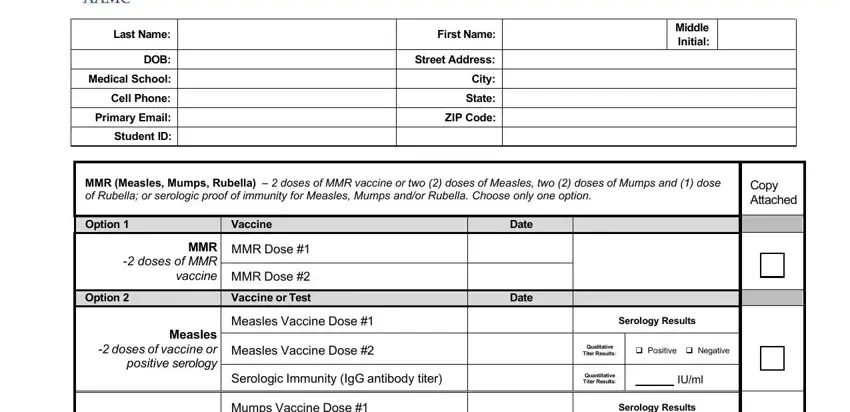
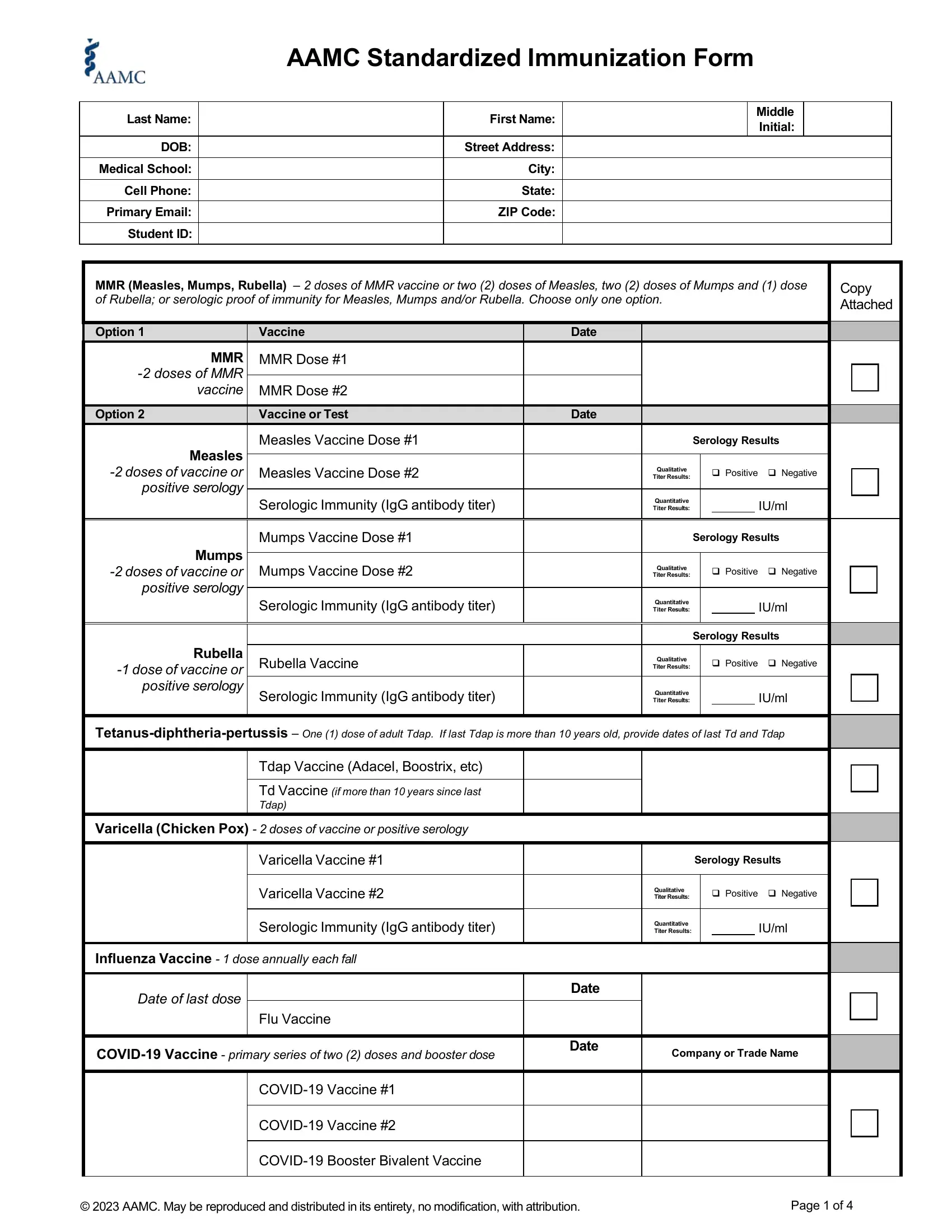
Inside the part Mumps doses of vaccine or, Mumps Vaccine Dose, Mumps Vaccine Dose, Serology Results, Qualitative Titer Results, Positive Negative, Rubella dose of vaccine or, Serologic Immunity IgG antibody, Quantitative Titer Results, IUml, Rubella Vaccine, Serology Results, Qualitative Titer Results, Positive Negative, and Serologic Immunity IgG antibody enter the particulars the program demands you to do.
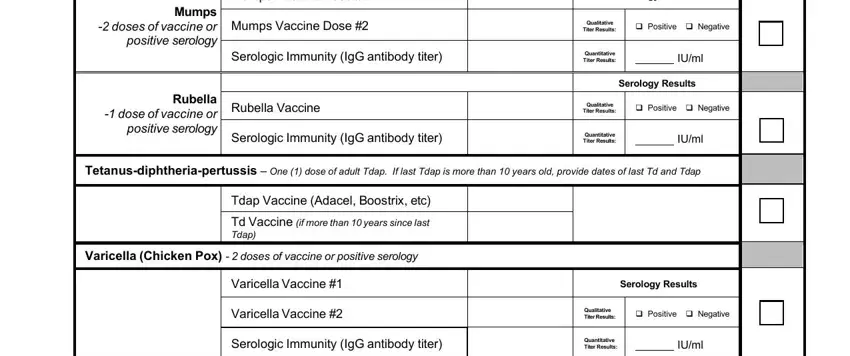
Write down the main details in Influenza Vaccine dose annually, Date of last dose, Flu Vaccine, COVID Vaccine primary series of, Date, Date, Company or Trade Name, COVID Vaccine, COVID Vaccine, COVID Booster Bivalent Vaccine, AAMC May be reproduced and, and Page of segment.
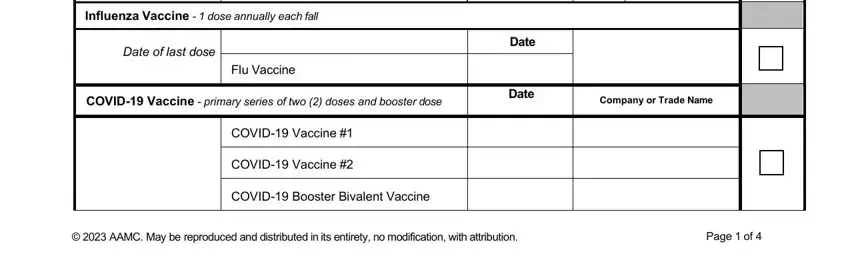
Identify the rights and responsibilities of the parties inside the part AAMC Standardized Immunization Form, Name, Last First Middle Initial, Date of Birth, mmddyyyy, Hepatitis B Vaccination doses of, Copy Attached, Primary Hepatitis B Series, HeplisavB only requires two doses, Additional doses of Hepatitis B, Only If no response to primary, HeplisavB only requires two doses, dose vaccines EnergixB PreHevbrio, Hepatitis B Vaccine Dose, and Hepatitis B Vaccine Dose.
End up by reading all these fields and preparing them as required: Vaccination Test or Examination, Date, Result or Interpretation, Physical Exam if required, AAMC May be reproduced and, and Page of.
Step 3: Click the Done button to ensure that your finished form could be transferred to any type of gadget you prefer or forwarded to an email you indicate.
Step 4: It could be safer to keep copies of your file. You can rest assured that we won't publish or check out your data.