application form for license to operate a medical x ray facility 2021 can be filled out online easily. Simply use FormsPal PDF tool to get it done quickly. FormsPal team is continuously working to expand the editor and insure that it is much easier for clients with its cutting-edge functions. Capitalize on the current progressive opportunities, and discover a trove of emerging experiences! Starting is effortless! Everything you should do is take the following easy steps directly below:
Step 1: Just hit the "Get Form Button" at the top of this site to open our pdf file editor. This way, you'll find everything that is needed to work with your file.
Step 2: As soon as you launch the editor, you will get the form all set to be filled in. In addition to filling out various blanks, you may as well do various other things with the file, such as writing custom textual content, modifying the initial textual content, inserting images, affixing your signature to the PDF, and more.
When it comes to blank fields of this particular document, here's what you want to do:
1. Before anything else, while completing the application form for license to operate a medical x ray facility 2021, begin with the area that features the next blank fields:

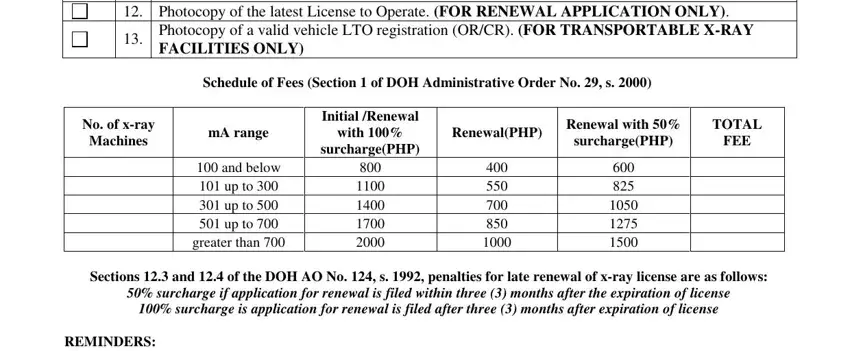
2. After the last part is completed, you need to put in the necessary details in License application fee refer to, Photocopy of the latest License, Photocopy of a valid vehicle LTO, Schedule of Fees Section of DOH, No of xray, Machines, mA range, Initial Renewal, with, surchargePHP, and below up to up to up to, greater than, RenewalPHP, Renewal with, and TOTAL so you can move forward further.
3. This next section will be focused on TYPE OF AUTHORIZATION, New application, Renewal of LTO, Amendment to existing LTO, II Name and qualifications of the, Head of the Facility Radiologist, FPCR Others, DPBR, SIGNATURE, Chief RadiologicXray Technologist, RRT, Radiation Protection Officer Name, SIGNATURE, MedicalHealth Physicist Name, and For CDRRHR use Doc Control No - fill out all these empty form fields.

4. Filling out Chief RadiologicXray Technologist, RRT, SIGNATURE, MedicalHealth Physicist Name, SIGNATURE if available, III Declaration of the veracity of, Remarks, Recommending Approval Date, Date, cid, Trunk Line local Telefax cid, Bldg San Lazaro Compound Rizal, and Page of is vital in this fourth form section - ensure to invest some time and fill in each and every empty field!

In terms of Chief RadiologicXray Technologist and Recommending Approval Date, be sure that you do everything correctly in this current part. Both these are considered the most significant fields in this file.
5. To wrap up your document, this final part requires several additional blanks. Filling out Type, Manufacturer, Tube head, Control Console, Tube head, Control Console, Tube head, Control Console, Max kVp, Max mA, Location, Please use separate sheet if, Radiography MobileStationary, Name and qualifications of other, and Name is going to wrap up everything and you can be done in a blink!
Step 3: After looking through your entries, click "Done" and you are good to go! Sign up with us right now and instantly obtain application form for license to operate a medical x ray facility 2021, prepared for downloading. Each modification you make is conveniently preserved , helping you to customize the file later on when necessary. FormsPal guarantees protected form completion without data record-keeping or any sort of sharing. Rest assured that your data is safe here!