Our PDF editor allows you to complete forms. It's not necessary to perform much to change hid forms alabama files. Simply check out the next actions.
Step 1: Choose the button "Get Form Here".
Step 2: The form editing page is now open. You can include text or manage present data.
The PDF file you wish to complete will contain the following areas:
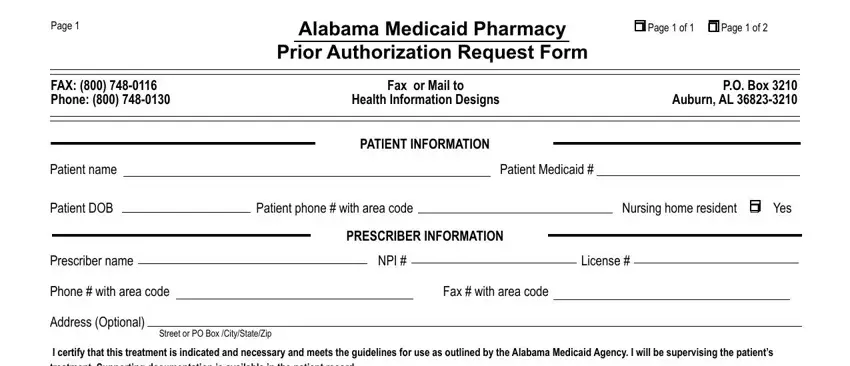
Put the necessary data in the CLINICAL INFORMATION, Strength, Qty, Days supply, PA Refills Other, Drug requested, J Code, If applicable, Diagnosis or ICDICD Code, Diagnosis or ICDICD Code, r Initial Request, r Renewal, r Maintenance Therapy r Acute, Medical justification, and r Additional medical justification box.
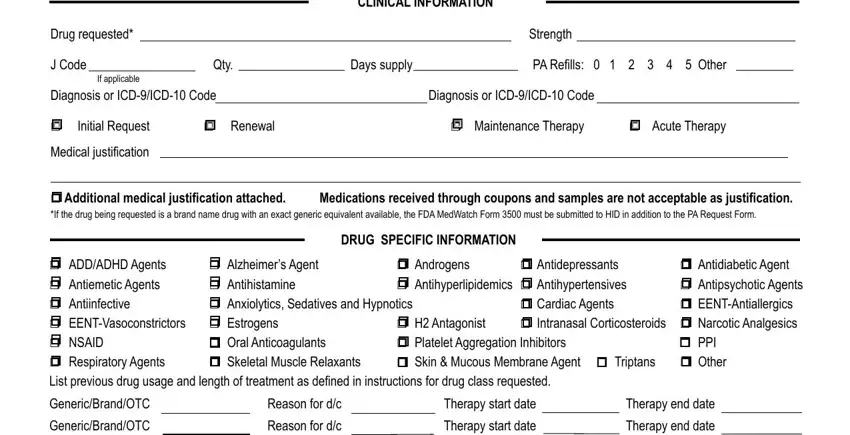
It is vital to provide certain particulars within the box Dispensing pharmacy, Phone with area code, NDC, DISPENSING PHARMACY INFORMATION, NPI, Fax with area code, NOTE See Instruction sheet for, and Alabama Medicaid Agency.

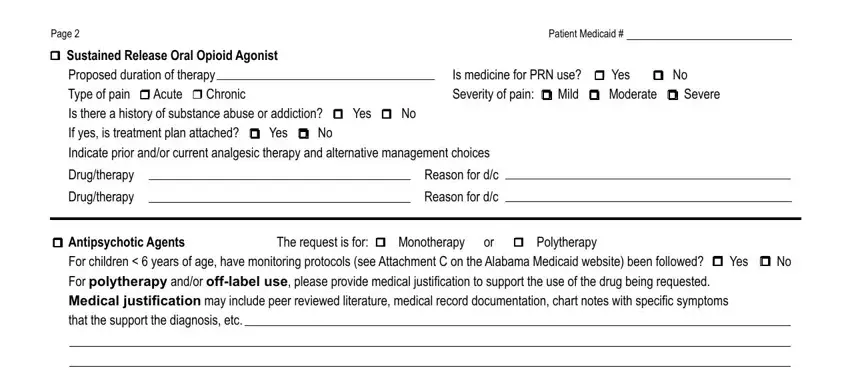
You have to describe the rights and obligations of each side in space Page, Patient Medicaid, r Sustained Release Oral Opioid, Is medicine for PRN use r Yes r No, Drugtherapy, Drugtherapy, Reason for dc, Reason for dc, r Antipsychotic Agents The request, and For children years of age have.
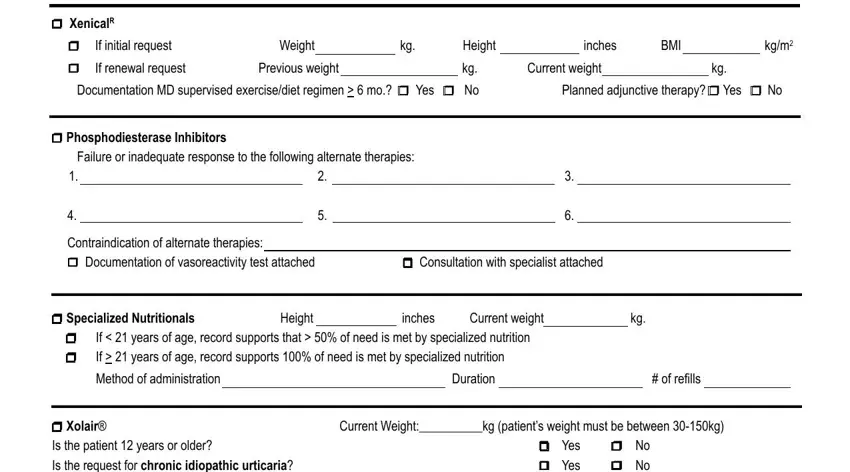
Fill in the form by analyzing these fields: r XenicalR, r If initial request Weight kg, inches BMI, kgm, r If renewal request Previous, Current weight, Documentation MD supervised, Planned adjunctive therapy r Yes r, r Phosphodiesterase Inhibitors, Failure or inadequate response to, Contraindication of alternate, r Documentation of vasoreactivity, r Consultation with specialist, r Specialized Nutritionals Height, and If years of age record supports.
Step 3: Once you click on the Done button, the finalized file is conveniently transferable to any type of of your devices. Or, you may send it via mail.
Step 4: Be sure to stay clear of possible future troubles by preparing at least a couple of duplicates of your document.