Due to the objective of allowing it to be as quick to use as it can be, we generated the PDF editor. The process of filling out the cigna prior authorization for remicade can be trouble-free should you try out the next steps.
Step 1: Click the button "Get Form Here".
Step 2: The file editing page is now open. Include text or enhance current details.
Enter the details requested by the software to create the form.
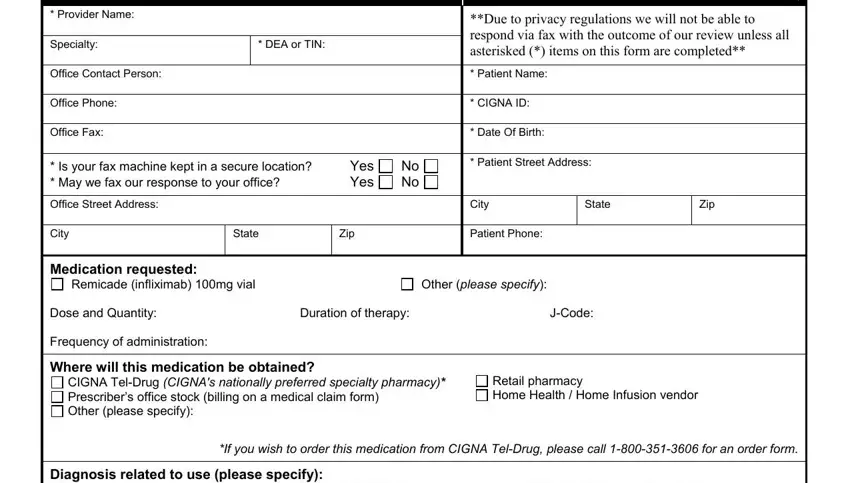
Fill in the Rheumatoid Arthritis Chronic, Psoriatic Arthritis Ulcerative, Active Ankylosing Spondylitis, What is the patients current weight, Has this patient been on Remicade, Yes, If YES what was the previous dosage, Does the patient have history of, Yes, Psoriatic or Reactive Arthritis, Yes, Rheumatoid Arthritis Will this, Yes, Please indicate if the patient has, and Methotrexate Penacillamine space with the information asked by the platform.
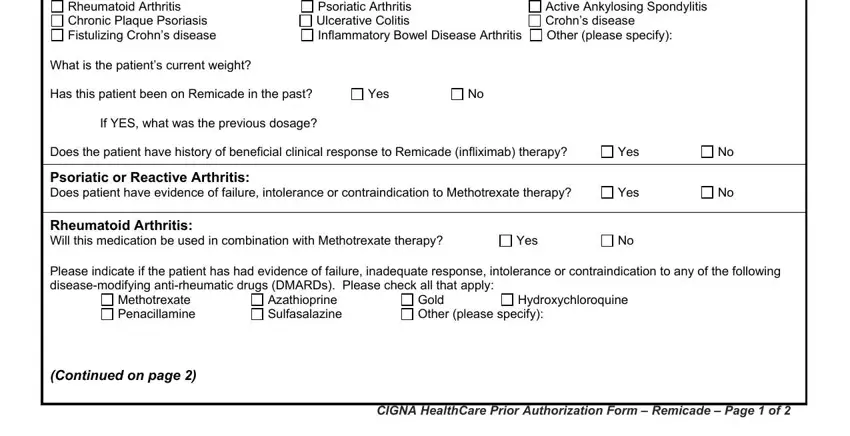
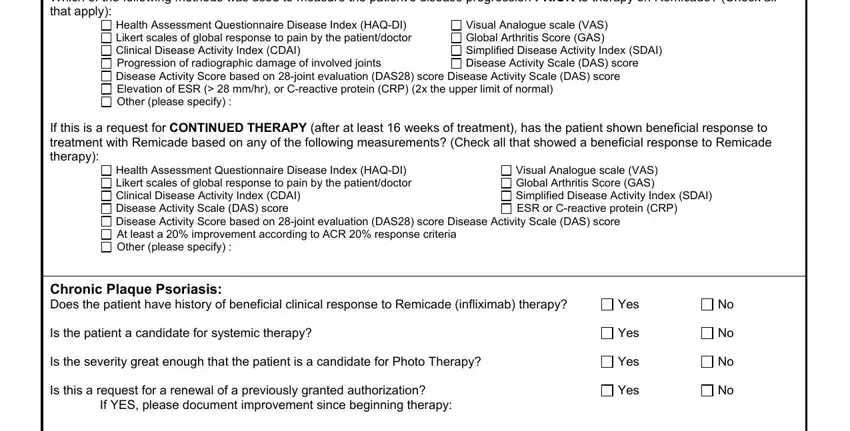
The system will require information to automatically fill up the segment Which of the following methods was, Health Assessment Questionnaire, Visual Analogue scale VAS Global, If this is a request for CONTINUED, Health Assessment Questionnaire, Visual Analogue scale VAS Global, Chronic Plaque Psoriasis Does the, Is the patient a candidate for, Is the severity great enough that, Is this a request for a renewal of, If YES please document improvement, Yes, Yes, Yes, and Yes.
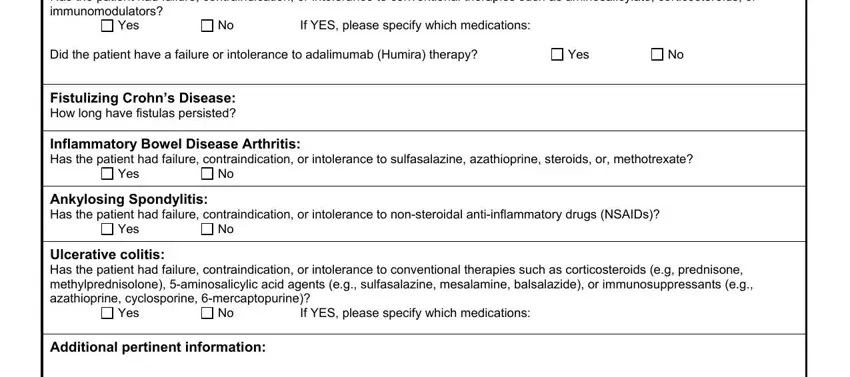
The Crohns Disease Has the patient had, Yes, If YES please specify which, Did the patient have a failure or, Yes, Fistulizing Crohns Disease How, Inflammatory Bowel Disease, Yes, Ankylosing Spondylitis Has the, Yes, Ulcerative colitis Has the patient, Yes, If YES please specify which, and Additional pertinent information section is going to be place to insert the rights and responsibilities of both sides.
Step 3: As soon as you select the Done button, your finished document can be simply transferred to each of your gadgets or to email specified by you.
Step 4: Be sure to create as many copies of your file as possible to avoid potential problems.


 No
No