Handling PDF documents online is definitely quite easy with this PDF editor. You can fill in arrival notice form here in a matter of minutes. We at FormsPal are aimed at giving you the best possible experience with our tool by constantly introducing new features and enhancements. With all of these updates, using our tool becomes better than ever! To begin your journey, go through these basic steps:
Step 1: Open the PDF file in our tool by pressing the "Get Form Button" in the top part of this webpage.
Step 2: With this advanced PDF editing tool, it is possible to accomplish more than merely complete blank form fields. Try each of the features and make your forms appear perfect with custom textual content added in, or adjust the original content to excellence - all supported by the capability to incorporate any kind of pictures and sign it off.
In order to complete this document, make sure that you provide the information you need in every single area:
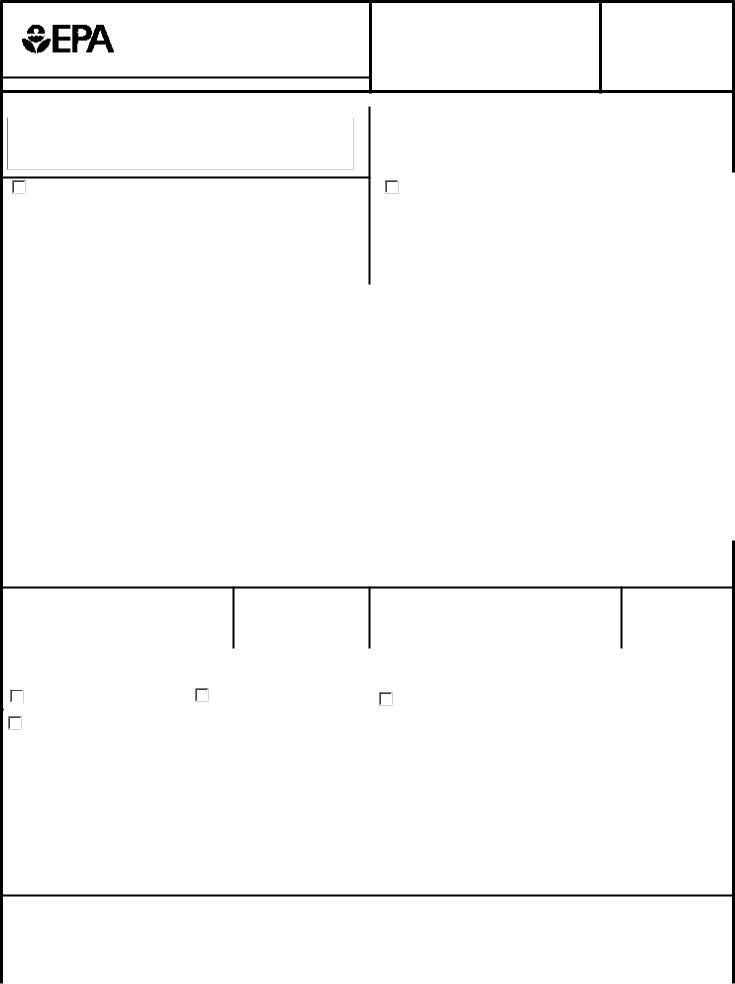
1. Whenever submitting the arrival notice form, be certain to complete all necessary blank fields in the associated area. This will help to expedite the process, allowing your information to be processed efficiently and properly.
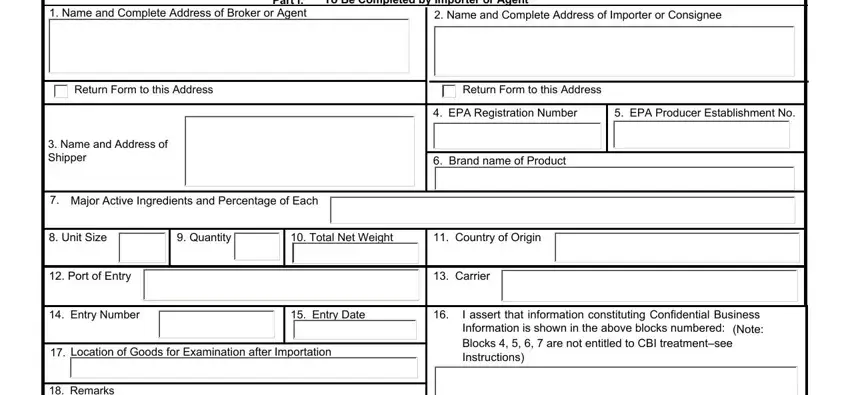
2. The subsequent part is usually to complete the next few blank fields: Remarks, I certify that the statements I, I acknowledge that any, Printed Name of Importer or Agent, Telephone Number, Signature of Importer or Agent, Date Signed, Certification, Part II, To Be Completed by US, Action to be taken by US Customs, Release Shipment, Detain for Inspection, Release shipment to consigne under, and Other.
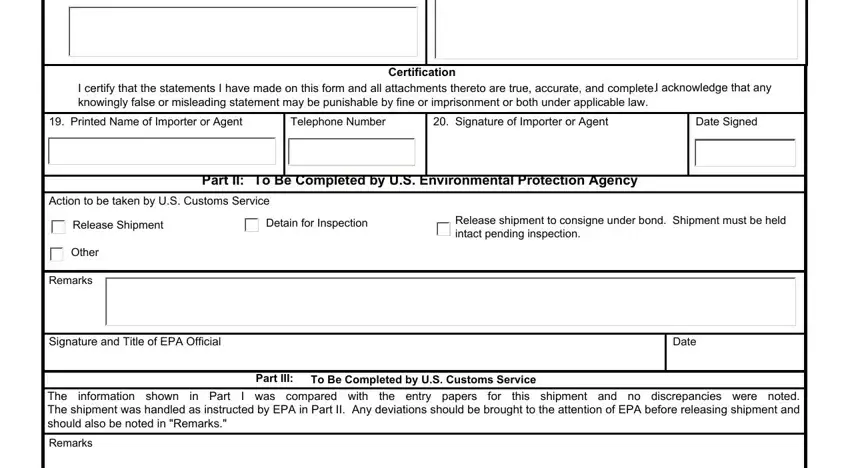
When it comes to Certification and Telephone Number, ensure that you review things in this section. These two are viewed as the most important ones in this page.
Step 3: Be certain that the information is accurate and then just click "Done" to complete the project. Join us right now and immediately gain access to arrival notice form, set for download. Every last change made is handily preserved , helping you to edit the form at a later point as required. FormsPal is dedicated to the privacy of our users; we always make sure that all information put into our tool is kept secure.