PAPERWORK REDUCTION ACT NOTICE and INSTRUCTIONS
PAPERWORK REDUCTION ACT NOTICE: Public reporting burden for this collection of information is estimated to average 0.85 hour per response, including time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. Send comments regarding the burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden, to Director, Collection
Strategies Division (2822T) U.S.Environmental Protection Agency,1200 Pennsylvania Ave, NW,Washington, DC20460.
INSTRUCTIONS: This form is to be used all applications for new registration, amendment, resubmission, to applications for notifications, final printed labeling, reregistration, etc. In order to process an application for a new registration submitted on this form, the following material must accompany the application:
1.Certification with Respect to Citation of Data (EPA Form 8570-34). [If not exempted by 40 CFR 152.81(b)(4)].
2.Confidential Statement of Formula (EPA Form 8570-4);
3.Formulator's Exemption Statement (EPA Form 8570-27);
4.Five copies of draft labeling;
5.Three copies of any data submitted;
6.Authorization letter where applicable;
7.Data Matrix.
Submission of Labeling -Labeling should first be submitted in the form of draft labeling with all applications. Such draft labels may be in the form of typed label text on 8.5 x 11 inch paper for submission or a mockup of the proposed label. If prepared for mockup, it should be constructed in a way as to facilitate storage in an 8.5 x 11 inch file. Mockup labels significantly smaller than 8.5 x 11 inches should be mounted on 8.5 x 11 inch paper for submission.
Submission of Data -Data submitted in support of this application must be submitted in accordance with PR Notice 86-5.
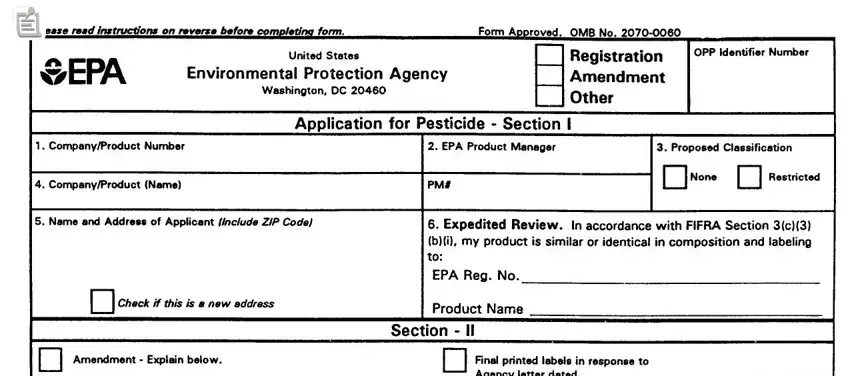
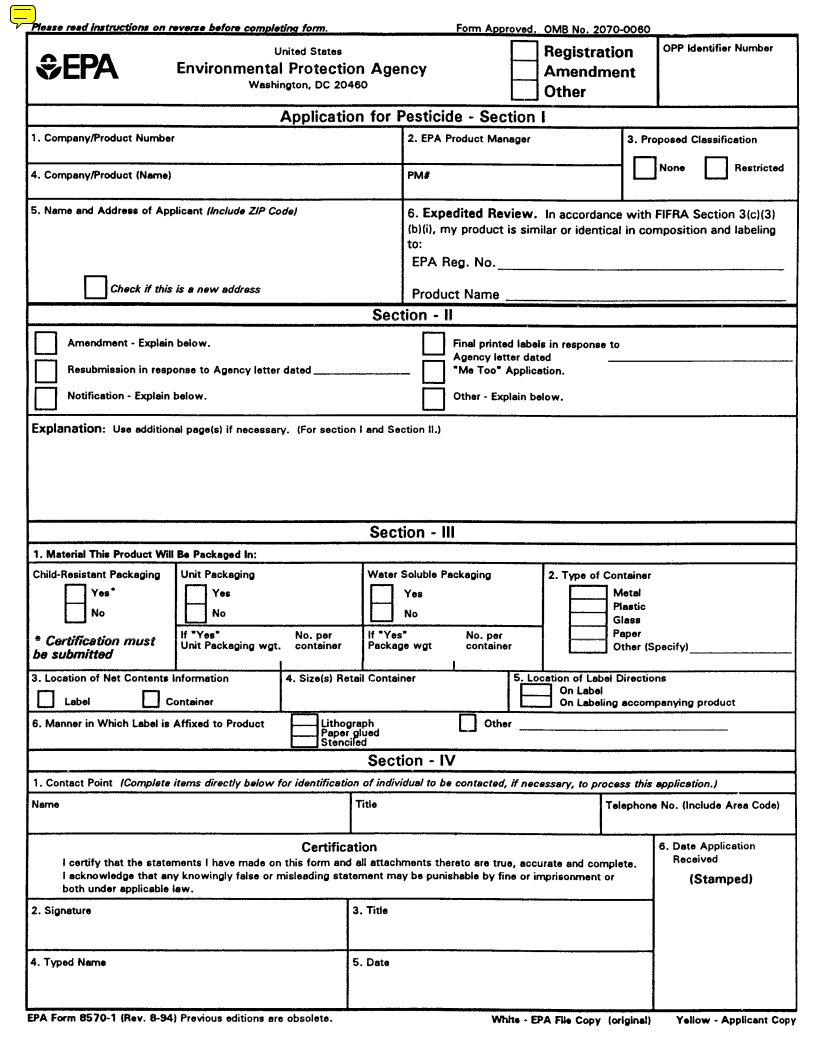
SPECIFIC INSTRUCTIONS: Please read the instructions listed below before completing this application. First determine the type of registration action, listed in Block A, for which you are submitting this application. For applications submitted in connection with new registration actions, Sections I, III, and IV must be completed by the applicant. For applications submitted in connection with amendments actions, resubmissions, notifications, reregistrations, etc., Sections I, II, and IV must be completed by the applicant.
Block A - Check the appropriate action for which you are submitting this form.
Section I - The section must be completed, as applicable, for all registration actions.
1.Company /Product Number - Insert your company number, if one has been assigned by EPA. This number rnay have been assigned to you as a basic registrant, a distributor, or as an establishment. If your product is registered, insert the Product Number.
2.EPA Product Manager -If known, fill in the name end PM number of the EPA Product Manager.
3.Proposed Classification -Specify the proposed classification of this product. For most products the classification would be “None”.
4.Product Name -Enter the complete product name of this pesticide as it will appear on the label. The name must be specific to this product only. Duplication of names is not permitted among products of the same company. Do not include any brand name or company line designations.
5.Name and Address of Applicant -The name of the firm or parson and address shown in your application is the person or firm to whom the registration will be issued. If you are acting on behalf of another party, you must submit authorization from that party to act for them in registration matters. An applicant not residing in theUnited Statesmust have an authorized agent residing in theUnited Statesto act for them in all registration matters. The name rand complete mailing address of such an agent must accompany this application.
6.Expedited Review -FlFRA section 3 (c) 3 (B) provides for expedited review of applications for registration, or amendments to existing registrations, that are similar or identical to other pesticide products that are currently registered with the EPA. In order for your application to be eligible for expedited review, you must provide us with the EPA Registration Number and product name of the product you believe is similar to or identical to your product. The product must be similar or identical in both formulation and labeled uses.
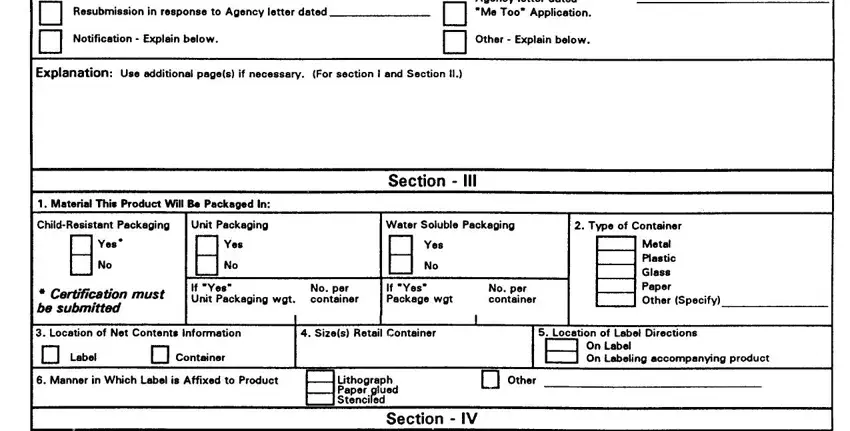
SECTION II -This section must be completed for a11 applications submitted to amend the registration only of a currently registered product (Amendment), for a resubmission in response to an Agency letter, for notifications to the Agency, for the submission of final printed labeling, for reregistration and for any other action that pertains to a specific EPA registered product. The Explanation Section should be used for any additional information regarding Sections I and II.
1.Subject of submission -Check the applicable block and provide the Agency letter date if appropriate. Provide a brief explanation of the purpose(s) for he submission, such as “the addition of a site, pest or crop (specify)”; “amend the Confidential Statement of Formula by…”; “reregistration submission”; “general label revision of direction for use”, `notification for…”. Attach a separate page if additional space is needed.
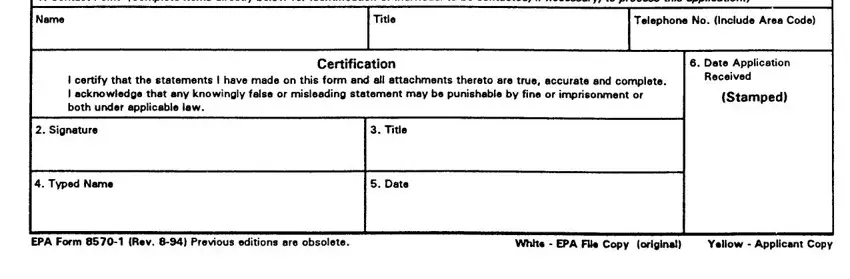
SECTION III - This Section must be completed for all applications submitted in connection with new registration or applicable amendments.
1.Type of Packaging -Check the appropriate block if your product will be packaged in the indicated packaging types. Indicate the size of the individual packets and number per retail container.
2.Type of Retail Container- Indicate type of container in which product will be marketed.
3.Location of Net Contents -Indicate the location of the net contents information for your product.
4.Size(s) of Retail Container -Specify the net contents of all retail containers for your product.
5.Location of Use Directions -Indicate the location of the use directions for your product.
6.Manner in which label is affixed to product -Indicated the method product label is attached to retail container.
SECTION IV (Contact Point) -This section must be completed for all applications for Registration actions, i.e., new products registration, resubmission, "me- too," reregistration, etc.
1-5.Self-explanatory
6. EPA Use Only