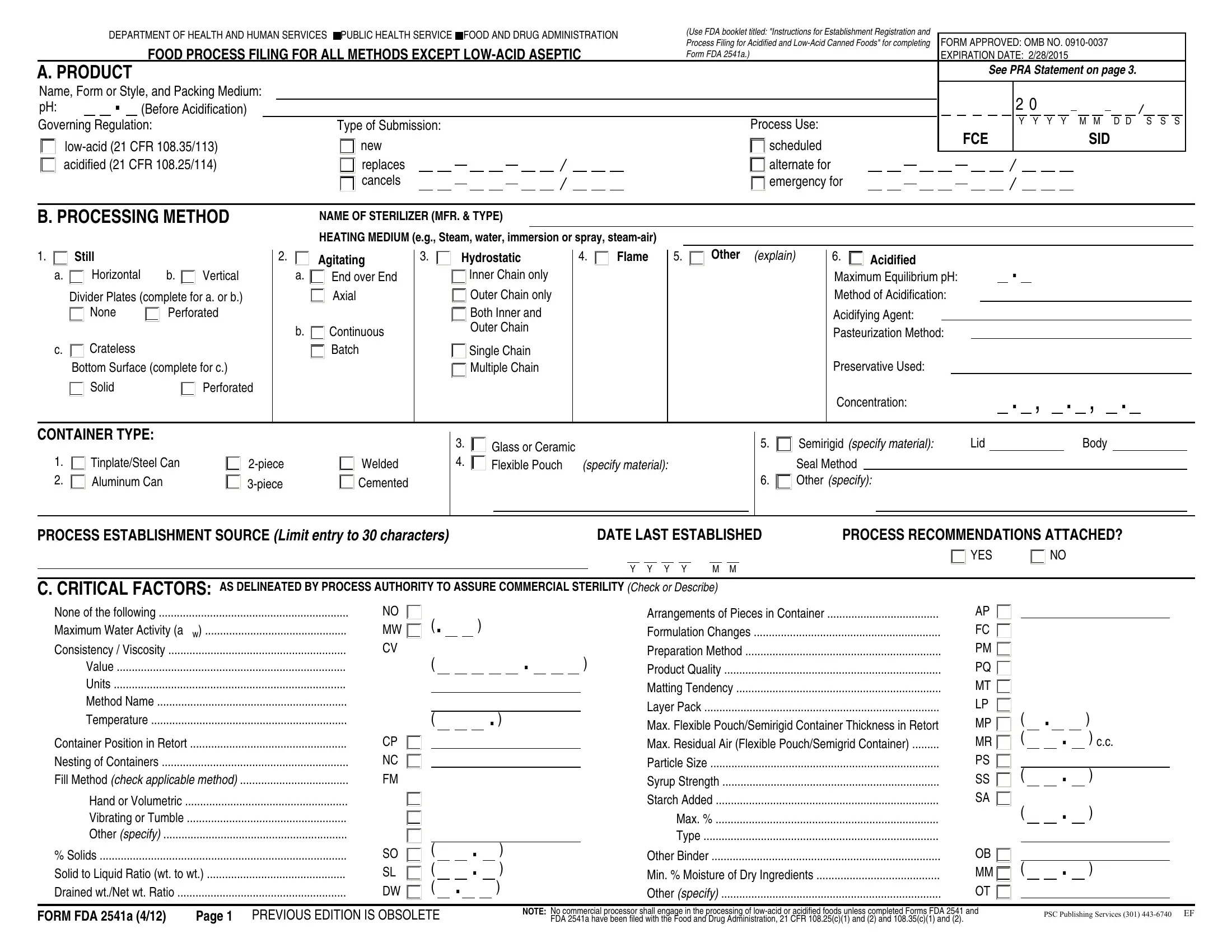
With the online PDF editor by FormsPal, you are able to complete or edit form fda food here. The tool is constantly upgraded by us, getting useful features and turning out to be more convenient. In case you are seeking to get going, here is what it requires:
Step 1: Press the "Get Form" button above on this page to access our PDF editor.
Step 2: The tool offers the capability to modify most PDF forms in a range of ways. Modify it with your own text, correct original content, and include a signature - all within several clicks!
As for the blanks of this particular document, here's what you need to do:
1. Start completing your form fda food with a number of essential blank fields. Gather all the important information and make certain not a single thing missed!
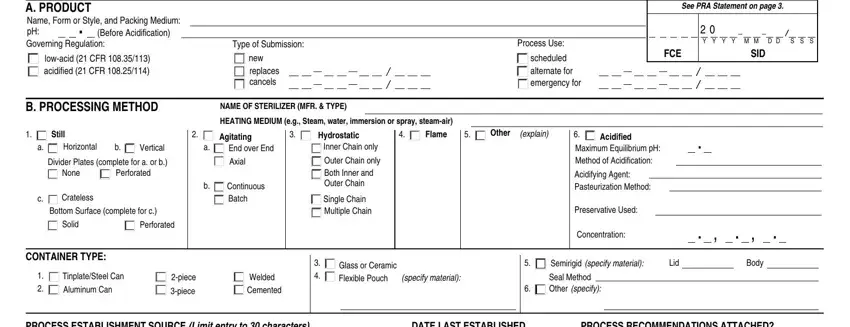
2. Once the previous segment is complete, you're ready insert the necessary details in PROCESS ESTABLISHMENT SOURCE Limit, DATE LAST ESTABLISHED, PROCESS RECOMMENDATIONS ATTACHED, YES, C CRITICAL FACTORS AS DELINEATED, None of the following Maximum, Container Position in Retort, Hand or Volumetric Vibrating or, Solids Solid to Liquid Ratio wt, NO MW CV, CP NC FM, SO SL DW, Arrangements of Pieces in, Other Binder Min Moisture of Dry, and AP FC PM PQ MT LP MP MR PS SS SA in order to move forward further.
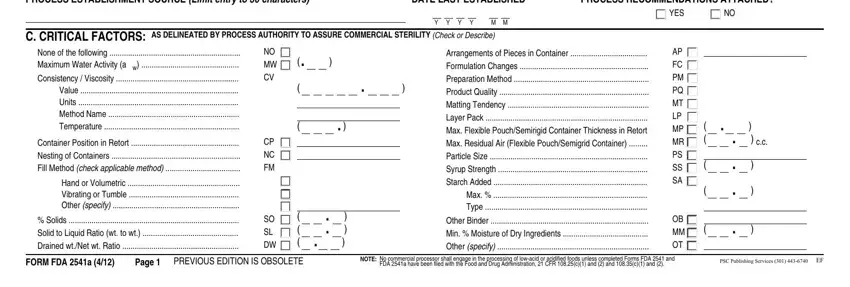
It's very easy to make an error while filling in the C CRITICAL FACTORS AS DELINEATED, consequently you'll want to take another look before you decide to submit it.
3. Within this step, have a look at D SCHEDULED PROCESS, Do not write in shaded areas, FCE, SID, CONTAINER DIMENSIONS, CAPACITY, UNITS, SCHEDULED PROCESS, Check Only One in Each Column, OTHER CRITICAL FACTORS TO ASSURE, COMMERCIAL STERILITY PER SOURCE, OTHER Specify, Step No, Temperature, and Process Time. Each of these need to be filled in with greatest precision.

4. Filling out FOR FDA USE ONLY, FULL NAME, Please Type or Print, TELEPHONE NUMBER, AUTHORIZED INDIVIDUAL, SIGNATURE, DATE, COMMENTS, PLANT NAME ADDRESS, PREFERRED MAILING ADDRESS, and FORM FDA a Page is crucial in this fourth stage - don't forget to take the time and be attentive with each blank area!
Step 3: Make certain the details are accurate and then click "Done" to proceed further. Get hold of your form fda food when you register online for a free trial. Instantly use the form inside your FormsPal account, with any modifications and changes being automatically saved! FormsPal guarantees protected form tools with no personal information recording or distributing. Be assured that your information is in good hands with us!