The Chh 704 form stands as a critical document in the management and treatment of patients undergoing outpatient hemodialysis, a life-sustaining procedure for individuals with severe kidney failure. This detailed form encapsulates a comprehensive dialysis prescription, including treatments per week, hours per treatment, and specifics regarding the dialysis solution used—dialysate temperature, potassium (K+), and calcium (Ca+) levels, alongside parameters for the dialyzer blood and dialysate flow. Moreover, it addresses the patient's dry weight, a vital metric for assessing fluid balance, and dosing for heparin, a medication essential for preventing blood clots during dialysis. Beyond dialysis specifics, the form encompasses diet recommendations, specifying protein, sodium, potassium, and fluid intakes, reflecting the tightly controlled dietary requirements for dialysis patients. It outlines the administration of heparin, including dosages and exceptions, vital sign monitoring protocols, and a comprehensive lab testing schedule to monitor the patient’s condition and treatment efficacy over time. Additionally, the form includes instructions for managing common complications associated with dialysis, such as hypotension and muscle cramps, and preventive measures like vaccinations. The remaining sections cover miscellaneous medical orders, from oxygen therapy to evaluations for kidney transplant eligibility, illustrating the form’s role in providing a holistic approach to patient care. By meticulously combining these elements, the Chh 704 form guides healthcare professionals in delivering standardized yet personalized dialysis treatment in an outpatient setting.
| Question | Answer |
|---|---|
| Form Name | Form Chh 704 |
| Form Length | 2 pages |
| Fillable? | No |
| Fillable fields | 0 |
| Avg. time to fill out | 30 sec |
| Other names | CHH 0704 Outpatient Hemodialysis Orders prime date dialysis form |
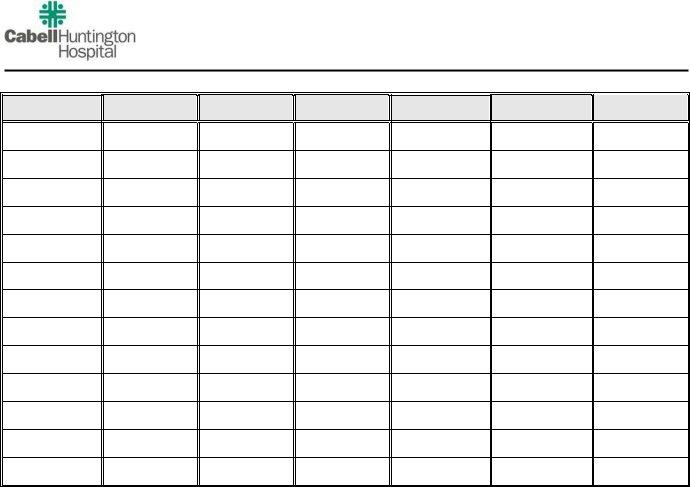
Outpatient Hemodialysis Orders
I.Dialysis Prescription
Treatments per Week
Hours per
Treatment
Dialysate Temperature
Dialysate K+
Dialysate Ca+
Dialyzer
Blood Flow
Dialysate Flow
Dry Weight
Heparin Bolus
Heparin Hourly Dose if on Pump
Heparin Prime
Date:
Date:
Date:
Date:
Date:
Date:
SIGNATURE
II. |
DIET: Protein_______Gm |
Na+_______gm K+_______mEq. Fluids__________ml/24 hrs |
III.HEPARIN:
A.Heparin Dialyzer prime to be diluted in 1000ml 0.9% normal saline.
B.Heparin bolus to be given through the venous line
C.Catheter: 5000units/ml Heparin to fill volume of catheter
D.INTERVENTIONAL RADIOLOGY or ACCESS SURGERY Patients:
1.FOR CATHETERS: Do not give Heparin on the day of placement, replacement and/or revision
2.FOR VASCULAR ACCESSES: DO give regular dose of Heparin on the day of procedure unless otherwise notified by radiology or surgery staff.
IV. |
VITAL SIGNS: |
A.Pre and post Dialysis: Temperature, B/P (sitting and standing) and pulse
B.Monitor B/P AND pulse a minimum of q 1 h.
V. LABORATORY:
A. ADMISSION:
B.WITHIN 1 WEEK OF ADMISSION: Pre/Post BUN for URR
C.MONTHLY:
D.QUARTERLY: Intact PTH; Hgb A1c (diabetes with renal manifestations) on all diabetics); Ferritin, Serum Iron, TIBC, Transferrin Saturation
E.
F.
G.ANNUALLY: HbsAB if antibody status is positive
H.PRN: Ferritin, Serum Iron, TIBC, Transferrin Saturation if Hgb less than 11 and it has been at least three months since last test; CRP for ferritins greater than or equal to 800 at individual physician discretion.
Patient Identification
Revised: 10/99, 6/0, 3/02, 5/03, 6/05, 6/06ss
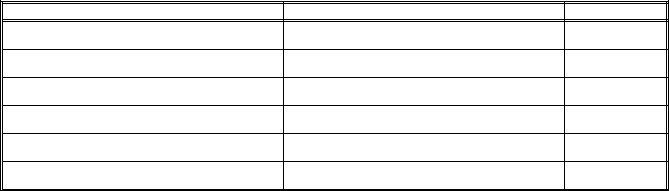
VI. |
HOME MEDICATIONS: |
|
|
A. |
Tylenol 650mg po q 4hrs. prn for pain |
|
B. |
Benadryl 25mg po q 6hrs. prn for itching |
|
C. |
Periactin 4mg po q 8hrs. prn for itching |
|
D. |
Pericolace 100mg 1 po BID prn for constipation |
|
E. |
Immodium 1 po TID prn for diarrhea |
VII. PRN MEDICATIONS:
A.Hypotension:
1.100ml bolus 0.9%NaCl IV. May Repeat as indicated if patient remains symptomatic
2.Albumin 25 gm IV for severe hypotension. May repeat X1 in 15minutes if patient remains hypotensive.
B.Muscle Cramps:
1.Concentrated Dextrose (50%) 50ml IVP for Nondiabetics.
2.Sodium profiling as indicated per extracorporeal system.
C.Seizure Activity:
1.Valium 2.5mg IV. May Repeat in 5 minutes if seizure activity persists.
2.Ativan 1mg IV. May repeat x2 every two minutes if seizure activity persists.
NOTE: NOTIFY M.D. IF ANY SEIZURE ACTIVITY OCCURS.
D.Nausea/Vomiting:
1.Phenergan 12.5mg IVP. May Repeat x 1 in 15 minutes if nausea or vomiting persists.
E.Hypoglycemia:
1.Dextrose 50% 25ml Bolus IVP. May repeat x 1 in 30 minutes if sugar remains below 60.
F.Chest Pain:
1.NTG 0.4mg SUBLINGUAL, may repeat every 5mins x 3, if chest pain persisits. If no relief, call MD.
G.Misc:
1.Benadryl 25mg IVP prn for itching or suspected drug reaction.
2.Acetaminophen 650mg po q 4hr prn pain.
H.Vaccines
1.Pneumonia Vaccine q 5 years.
2.Flu Vaccine annually
3.Hepatitis B Vaccine per protocol if antibody negative (Hepatitis status due to CKD- End stage renal disease) Note: Documentation of patient refusal for any of the above should be noted on the flu vaccination form and placed on the patient’s chart.
VIII. MISC:
A.Oxygen @2L/min PRN for Chest Pain or dyspnea.
B.Culture all wound/catheter site drainage PRN and notify MD for orders.
C.Glucoscans as indicated.
D.Epogen / Procrit / Aranesp per protocol ( Anemia, unspecified).
E.Ferrlecit per protocol (Iron deficiency, Anemia, unspecified).
F.Blood Cultures X 2 PRN temperature greater than 101 degrees F, obtaining one from access and one peripherally
(NOTIFY M.D. FOR FURTHER ORDERS).
G.CXR as indicated for Estimated Dry Weight adjustment per charge nurse (Fluid overload).
H.Hectoral / Zemplar per protocol (secondary hyperparathyroidism of renal origin).
I.TPA 2mg to each limb to dwell
J.Transplant Coordinator evaluate for patient’s interest in transplant, refer to appropriate agencies, and document.
K.Transplant Coordinator evaluate for permanent access placement, refer to appropriate agencies, and document.
L.Reprocessing of dialyzer if patient consents.
M.Tuberculosis skin test, if not allergic, as needed for travel.
PHYSICIAN SIGNATURE
R.N. SIGNATURE
DATE
Patient Identification |
|
Revised: 10/99, 6/0, 3/02, 5/03, 6/05, 6/06ss |
|