By using the online PDF editor by FormsPal, you are able to fill in or change RELABEL right here. In order to make our editor better and simpler to use, we continuously work on new features, with our users' feedback in mind. Getting underway is effortless! All that you should do is stick to the next basic steps directly below:
Step 1: Firstly, access the pdf tool by pressing the "Get Form Button" in the top section of this site.
Step 2: After you open the PDF editor, you will find the form all set to be filled out. Other than filling out different blank fields, you may also perform some other actions with the PDF, particularly adding any words, modifying the original text, adding images, putting your signature on the form, and more.
So as to fill out this PDF form, make certain you provide the necessary details in every single blank field:
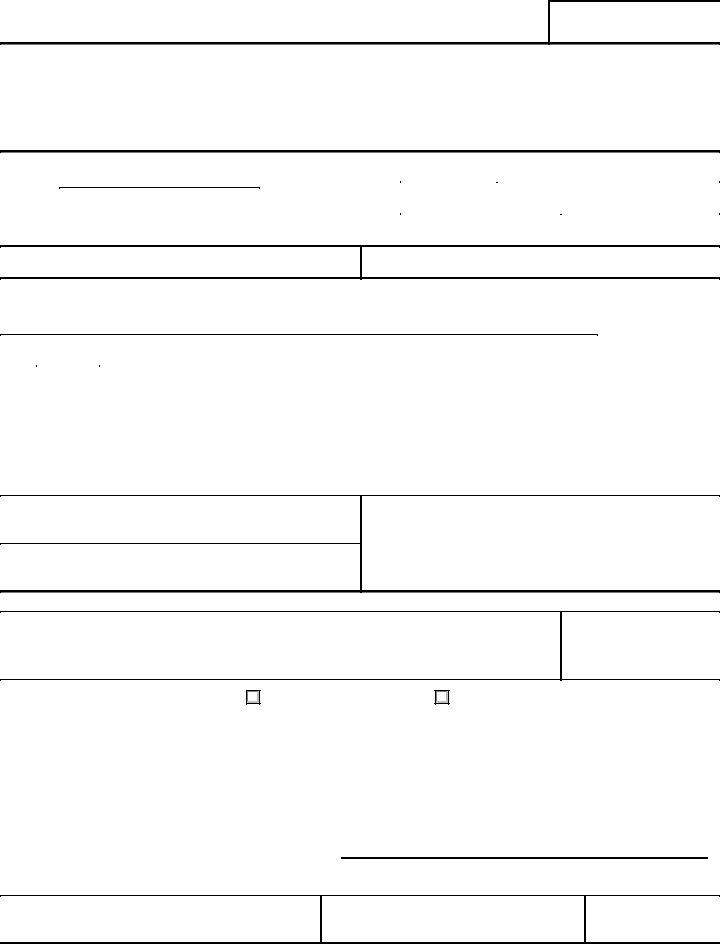
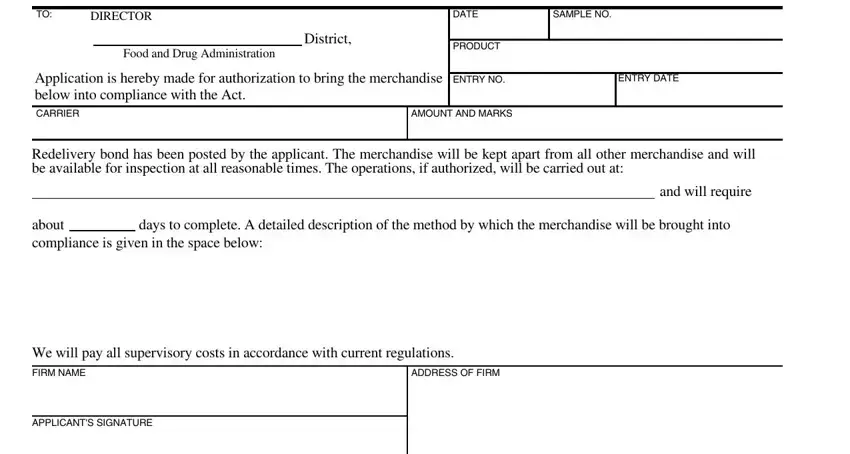
1. It is advisable to complete the RELABEL accurately, hence take care while filling in the parts comprising these specific fields:
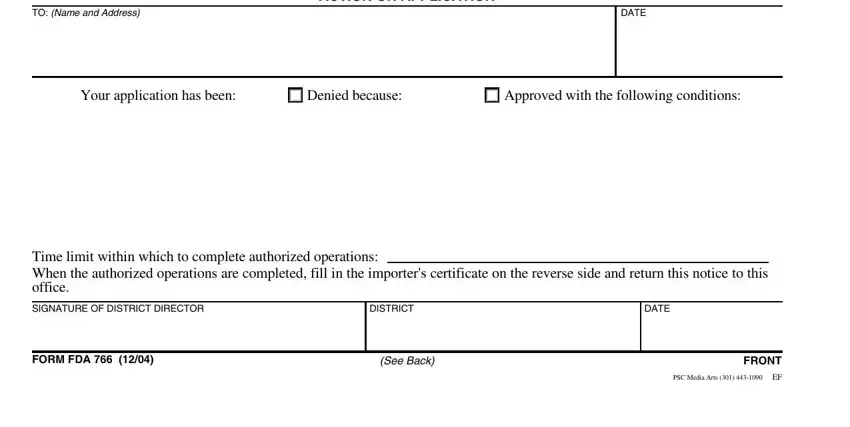
2. Your next step would be to submit all of the following fields: TO Name and Address, DATE, ACTION ON APPLICATION, Your application has been, Denied because, Approved with the following, Time limit within which to, SIGNATURE OF DISTRICT DIRECTOR, DISTRICT, DATE, FORM FDA, See Back, FRONT, and PSC Media Arts.
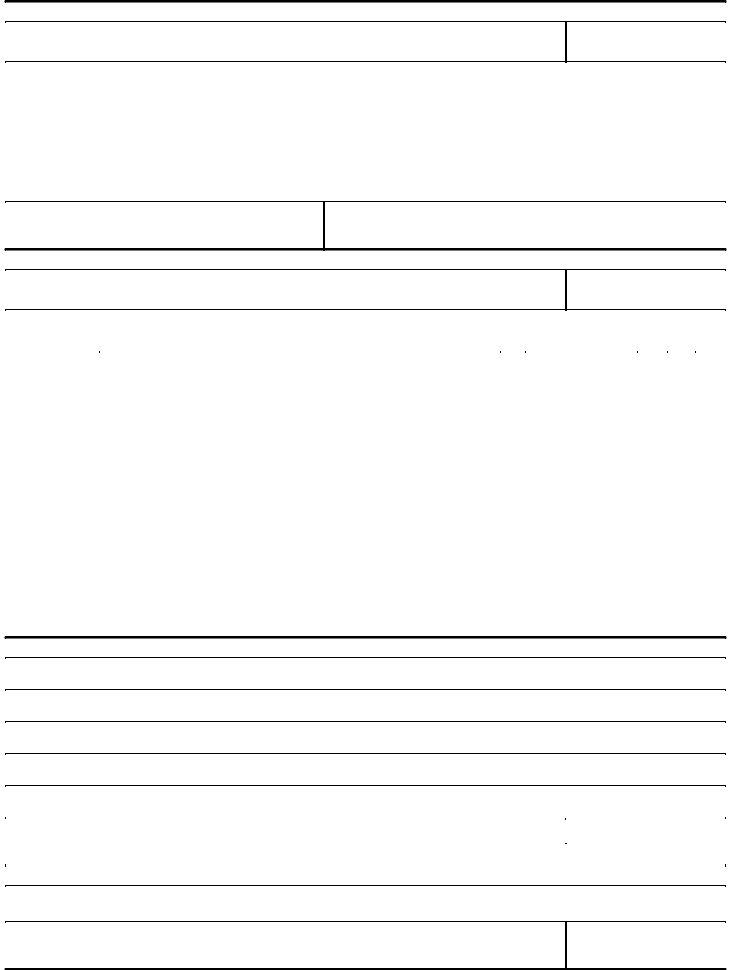
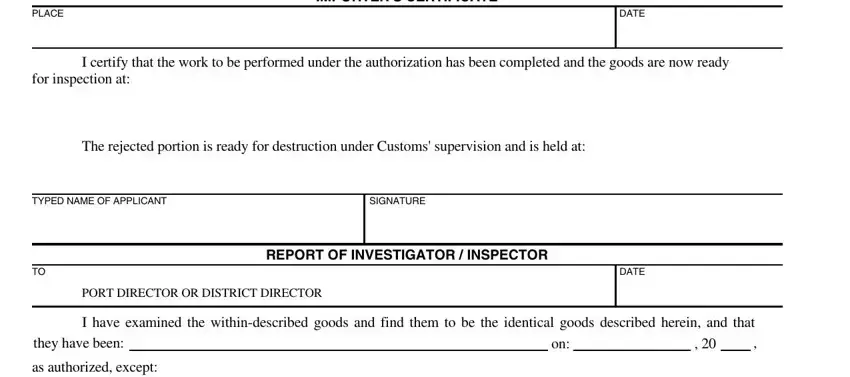
3. This stage is going to be straightforward - fill out all of the form fields in PLACE, DATE, IMPORTERS CERTIFICATE, I certify that the work to be, for inspection at, The rejected portion is ready for, TYPED NAME OF APPLICANT, SIGNATURE, DATE, REPORT OF INVESTIGATOR INSPECTOR, PORT DIRECTOR OR DISTRICT DIRECTOR, I have examined the, they have been, and as authorized except to complete this part.
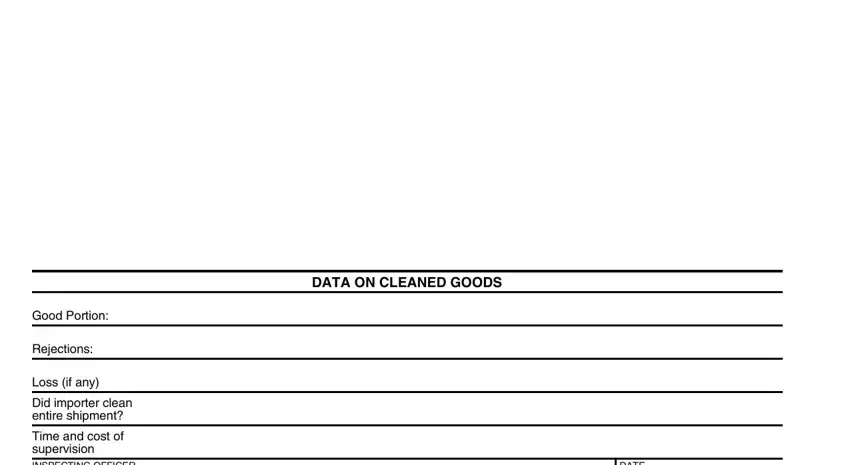
4. Now fill out this next part! In this case you've got all these DATA ON CLEANED GOODS, Good Portion, Rejections, Loss if any, Did importer clean entire shipment, Time and cost of supervision, and DATE form blanks to fill in.
5. The very last step to complete this PDF form is critical. Be certain to fill out the appropriate fields, like Time and cost of supervision, Disposed of as noted above, DIRECTOR OF CUSTOMS, DIRECTOR OF DISTRICT, DATE, DATE, FORM FDA, and BACK, prior to finalizing. If not, it can contribute to a flawed and possibly incorrect paper!
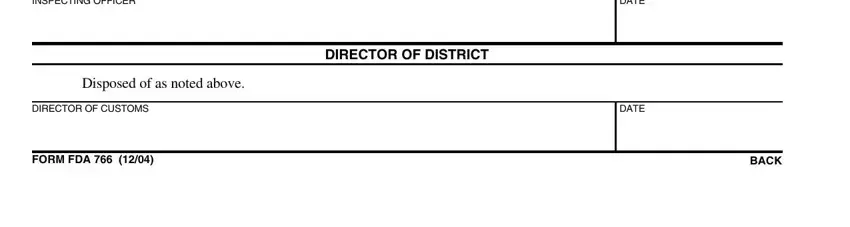
Be really careful when filling in DIRECTOR OF CUSTOMS and Disposed of as noted above, as this is the section in which a lot of people make mistakes.
Step 3: Soon after looking through the entries, click "Done" and you are done and dusted! Get hold of your RELABEL once you register here for a 7-day free trial. Immediately gain access to the document inside your FormsPal account, together with any edits and changes being automatically kept! When using FormsPal, you're able to complete documents without the need to be concerned about information breaches or records being distributed. Our secure software ensures that your personal data is maintained safely.