Having the purpose of allowing it to be as effortless to work with as it can be, we built this PDF editor. The entire process of filling up the prime therapeutics prior authorization forms is going to be straightforward when you follow the next actions.
Step 1: Choose the orange button "Get Form Here" on the website page.
Step 2: The file editing page is currently available. You can add text or change current content.
The next parts will make up the PDF template that you'll be filling out:
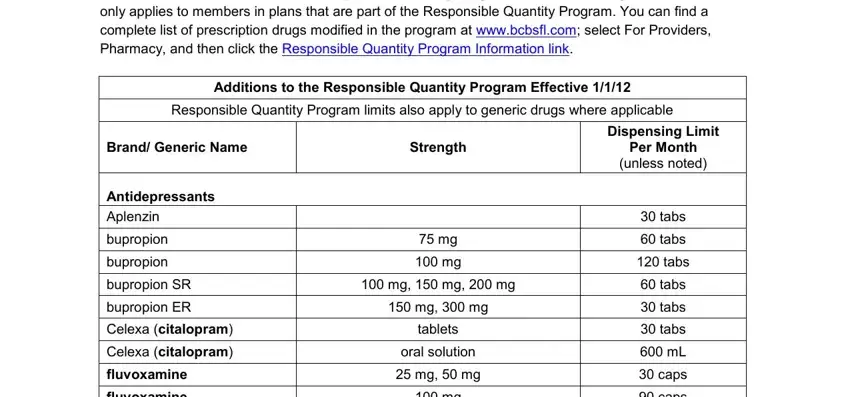
Include the expected details in the This program ensures coverage of, Additions to the Responsible, Responsible Quantity Program, Brand Generic Name, Strength, Dispensing Limit Per Month unless, Antidepressants Aplenzin, bupropion, bupropion, bupropion SR, bupropion ER, Celexa citalopram, Celexa citalopram, fluvoxamine, and fluvoxamine area.
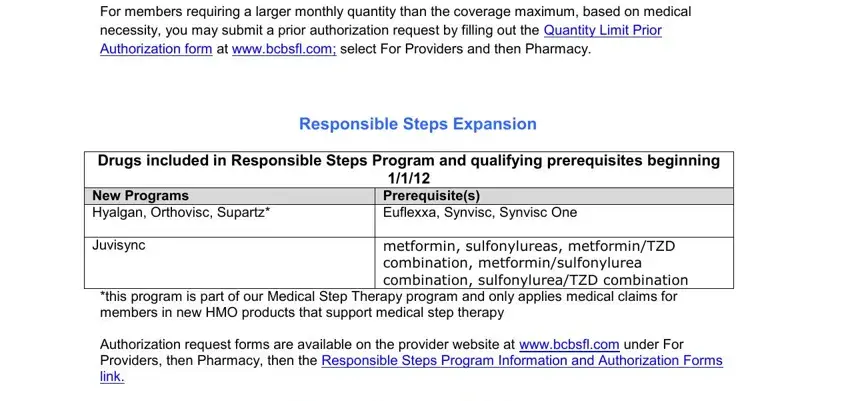
Point out the most crucial information on the For members requiring a larger, Responsible Steps Expansion, Drugs included in Responsible, New Programs Hyalgan Orthovisc, Juvisync, metformin sulfonylureas, this program is part of our, and Authorization request forms are segment.
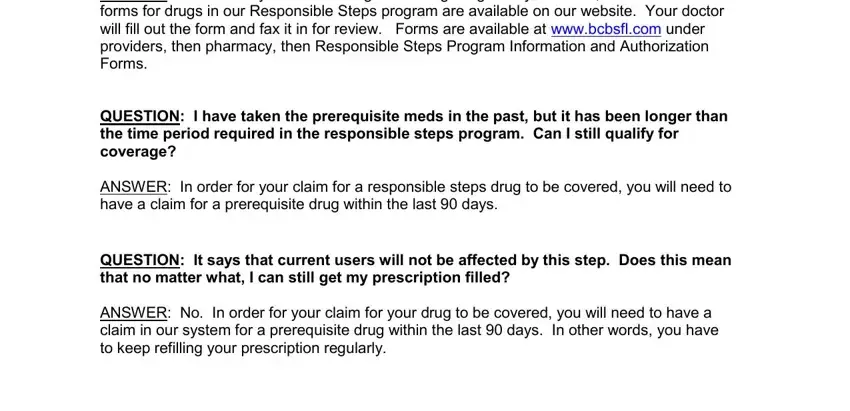
Please include the rights and responsibilities of the sides in the ANSWER Check with your doctor if a, QUESTION I have taken the, ANSWER In order for your claim for, QUESTION It says that current, and ANSWER No In order for your claim box.
Step 3: Click the Done button to save the document. Now it is obtainable for transfer to your electronic device.
Step 4: To prevent yourself from any kind of risks as time goes on, try to make at least several duplicates of the form.