In today's complex consumer market, proactive management of product recalls and withdrawals is essential to safeguard public health and preserve brand integrity. The Recall Procedure Flow Chart form serves as a critical tool in this endeavor, guiding stakeholders through the nuanced process of managing a product issue that could pose a health risk. The form outlines a distinct pathway for assessing the risk associated with a product, advising manufacturers or suppliers of the risk for corporate brands, and determining the appropriate course of action. Importantly, it differentiates between situations that necessitate a full product recall versus those that may only require a withdrawal, underscoring the importance of a precise response tailored to the severity of the risk. The form further delineates the roles of various parties involved—from manufacturers and suppliers to retailers, government authorities, and even consumers—in identifying potential recalls or withdrawals. This includes the initial notification process, convening a product crisis management meeting if necessary, and the steps required for both quality assurance and engaging with regulatory bodies and the public. This comprehensive approach ensures that all stakeholders are prepared to act swiftly and effectively, minimizing health risks to consumers and facilitating the prompt resolution of any issues that arise. In essence, the Recall Procedure Flow Chart form encapsulates a structured, collaborative response to product safety concerns, ensuring transparency, accountability, and the maintenance of consumer trust.
| Question | Answer |
|---|---|
| Form Name | Recall Procedure Flow Chart Form |
| Form Length | 1 pages |
| Fillable? | No |
| Fillable fields | 0 |
| Avg. time to fill out | 15 sec |
| Other names | recall plan examples, example of process flow diagram, warehouse flowchart in words, recall plan flow chart |
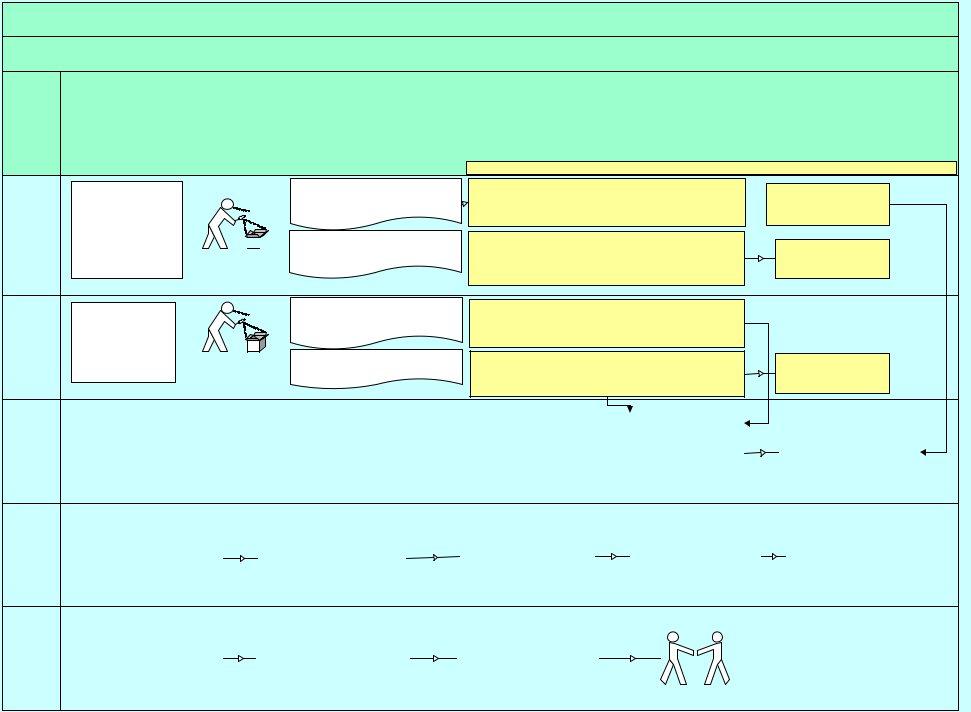
Product Recall & Withdrawal Process Flowchart
Recall:
Withdrawal:
Initial |
Notification |
|
|
If health risk (i.e. serious illness, injury or death) then a Trade or consumer level recall is initiated. Adverts for Consumer level recalls must be seen by agency for approval ! If product is
A potential recall or withdrawal may be identified or advised through the actions of the following:
-Manufacturer/Suppliers own QA
-Retailers own reports from complaints
-Third Party or Second Party Auditors
-Health Officials and/or Regulatory Bodies e.g. FSANZ, NZFSA testing
-Consumers via complaints
-Police
Corrective Action
Manufacturers / |
Suppliers |
|
|
If No Risk - terminate process
If Minor or Quality risk (i.e. no health risk) - withdrawal adequate
If Health Risk identified - go to next Quality Assurance step for Product Recall
and Data collection to determine if any
Quality or Consumer Safety risk
No Risk to consumer safety. Notify verbally and with
submission of completed Product Recall / Withdrawal Form to:
-Retailer ,Other Trade customers, Warehouse/DC, Govt Authorities as applicable
Risk to consumer safety confirmed - Recall required. Notify verbally and with submission of completed Product Recall / Withdrawal Form to: Government Authority, Retailer, Other Trade customers, Warehouse/DC
Notify the retailers of when
compliant stock is
available for restocking
Notify media and public (if consumer level recall)
Retailer |
Corporate |
Brands |
|
|
|
Quality Assurance and Data collection to determine if any
Quality or Consumer Safety risk
No Risk to consumer safety. Notify verbally and with submission of
completed Product Recall / Withdrawal Form to: Retailers ,Other Trade
customers, Warehouse/DC, Govt Authorities as applicable
Risk to consumer safety confirmed - Recall required. Notify verbally and
with submission of completed Product Recall / Withdrawal Form to:
Government Authority, Retailer, Other Trade customer, Warehouse DC
Notify media and public (if consumer level recall)
|
Retailer |
|
|
Government |
Authorities |
||
Recall / |
Withdrawal |
Review |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Retailer to advise manufacturer |
||
|
|
|
|
|
|
|
Retailer to receive verbal and/or written notification via |
|
|
of requirements for how and |
|||||
|
|
|
|
|
|
|
Product Recall / Withdrawal Form and to agree to |
|
|
when fresh product can |
|||||
|
|
|
|
|
|
|
corrective action processes e.g. product returns |
|
recommence to be distributed |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
for sale again |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Manufacturer or Retailer to |
|
|
|
Retailer & Manufacturer |
|
|
Notify all relevant |
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|||||
If risk to public safety confirmed |
|
|
|
|
Discontinue distribution of |
|
|
regulatory agencies, |
|
|
Notify public |
|
|||
|
supply A&NZ Product Recall |
|
|
|
|
|
|
|
|
||||||
a recall is required. |
|
|
|
|
|
product and removal of |
|
|
wholesale and other trade |
|
|
(if necessary) |
|
||
|
Withdrawal Form |
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
product from sale |
|
|
customers |
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Manufacturer/Supplier instigated |
|
Manufacturers/Suppliers, Retailers |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
and Government authorities to |
|
|
Key stakeholders distribute |
|
|
|
|
|
|
|
|
|||
recall / withdrawal - prepare |
|
|
|
|
|
|
|
|
|
|
|
||||
|
monitor completion and |
|
|
|
summaries and |
|
|
|
|
|
|
|
|
||
summary and recommendations |
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
effectiveness of recall or |
|
|
|
recommendations |
|
|
|
|
|
|
|
|
||
as required |
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
withdrawal |
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
Quality Assurance Confidence |
|
|
|
|||