It's a breeze to complete the plan medication form. Our PDF tool was designed to be easy-to-use and allow you to fill out any PDF efficiently. These are the basic steps to go through:
Step 1: Locate the button "Get Form Here" and press it.
Step 2: It's now possible to edit the plan medication form. The multifunctional toolbar permits you to add, delete, change, and highlight content or perform other sorts of commands.
If you want to complete the plan medication form PDF, enter the content for all of the sections:
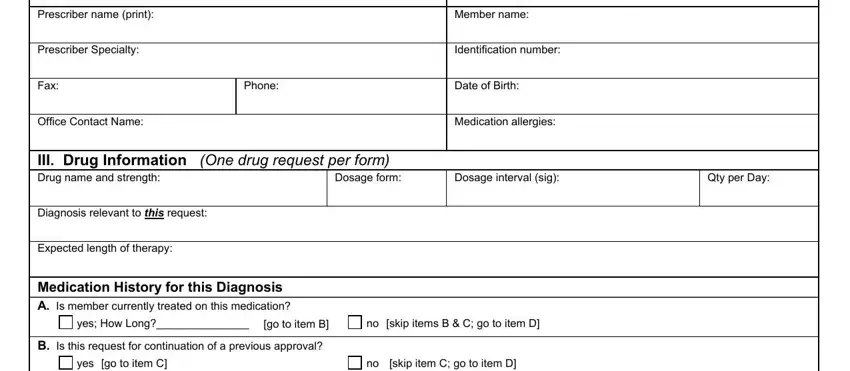
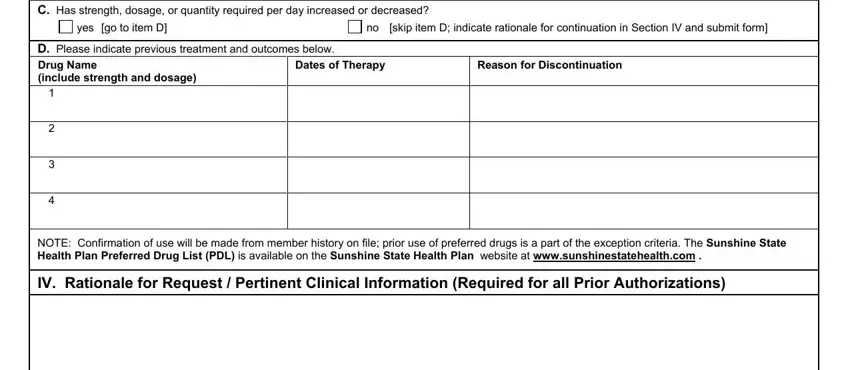
Complete the C Has strength dosage or quantity, yes go to item D, no skip item D indicate rationale, D Please indicate previous, Dates of Therapy, Reason for Discontinuation, NOTE Confirmation of use will be, and IV Rationale for Request section with all the details asked by the application.
The system will request for more info to automatically prepare the area Appropriate clinical information, Provider Signature, Date, US Script will respond via fax or, and Contact Caremark at for.

Step 3: Press the Done button to save the document. So now it is readily available for export to your gadget.
Step 4: It may be simpler to save duplicates of the form. You can be sure that we are not going to disclose or check out your information.


