Using PDF forms online is actually very simple with our PDF editor. You can fill in dea 252 form here painlessly. We are aimed at giving you the ideal experience with our tool by regularly adding new features and improvements. Our tool is now much more useful as the result of the newest updates! At this point, filling out PDF documents is a lot easier and faster than before. Getting underway is easy! All you need to do is follow these basic steps down below:
Step 1: Hit the "Get Form" button above on this page to open our editor.
Step 2: This tool offers you the capability to customize the majority of PDF documents in a variety of ways. Change it with any text, adjust existing content, and add a signature - all at your fingertips!
Filling out this PDF requires thoroughness. Ensure every single blank is filled in accurately.
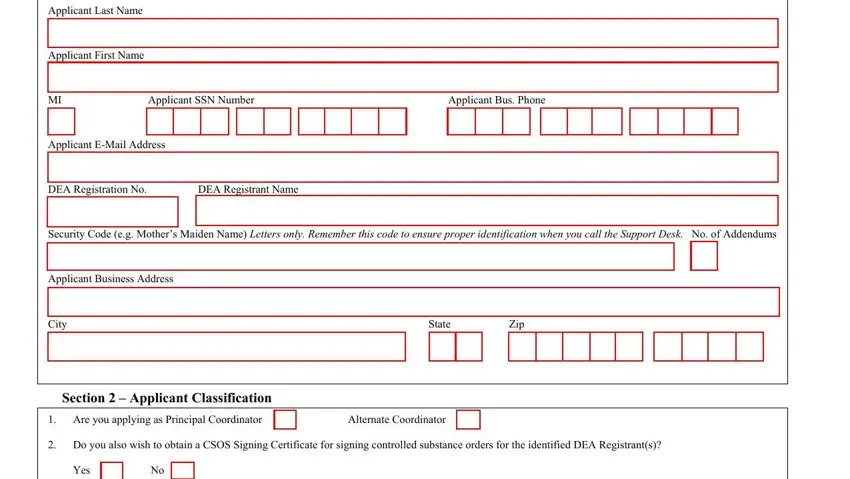
1. First, while filling in the dea 252 form, start in the part that contains the following fields:
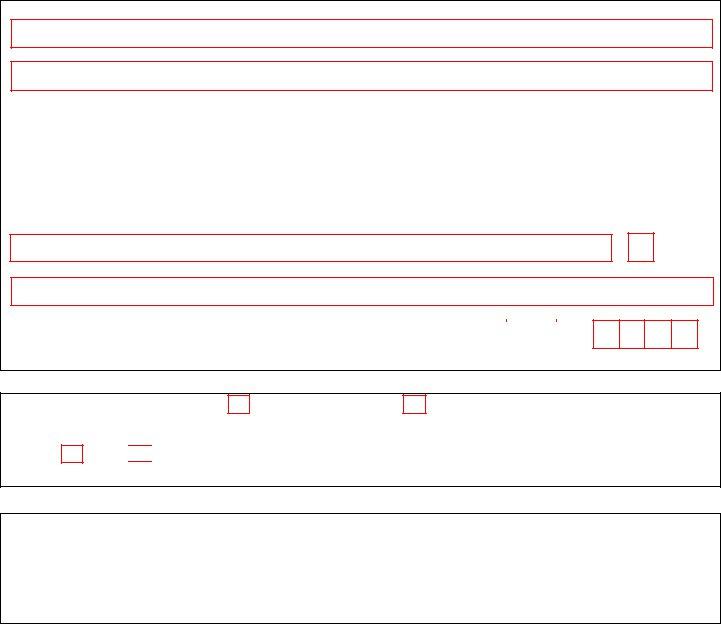
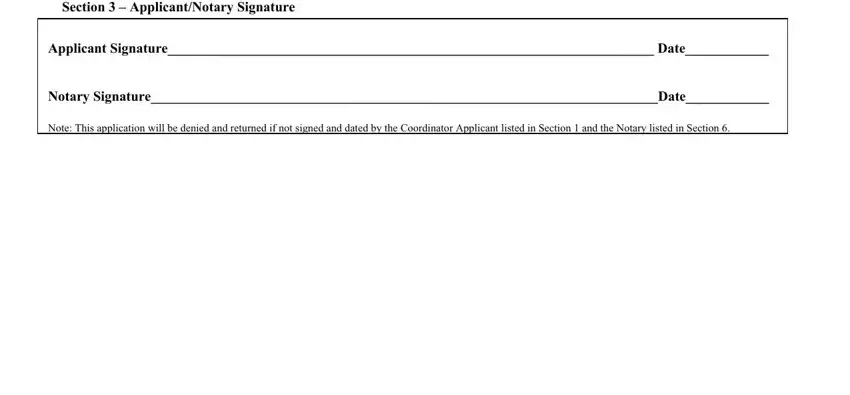
2. Once your current task is complete, take the next step – fill out all of these fields - Section ApplicantNotary Signature, Applicant Signature Date, Notary SignatureDate, and Note This application will be with their corresponding information. Make sure to double check that everything has been entered correctly before continuing!
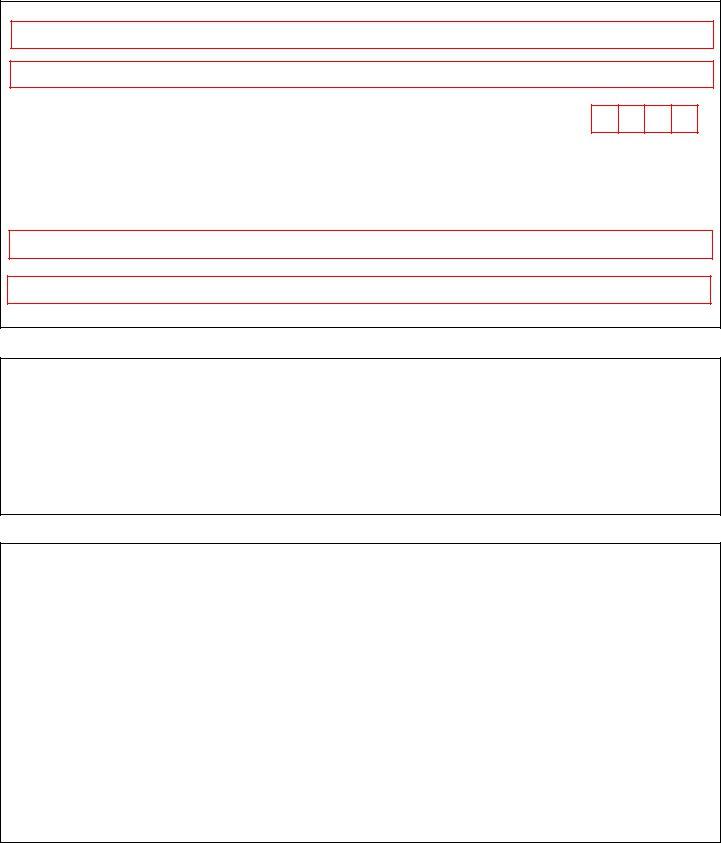
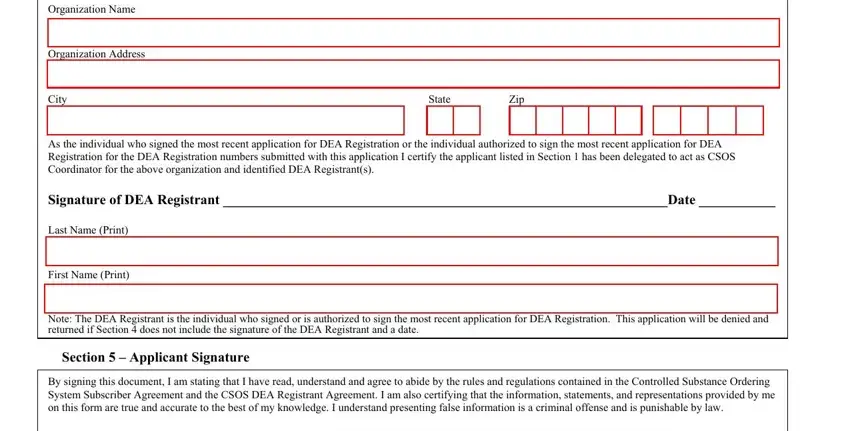
3. Completing Organization Name, Organization Address, City, State, Zip, As the individual who signed the, Signature of DEA Registrant Date, Last Name Print, First Name Print, Section Applicant Signature, Note The DEA Registrant is the, Section Applicant Signature, and By signing this document I am is essential for the next step, make sure to fill them out in their entirety. Don't miss any details!
4. This particular paragraph comes next with the following blank fields to fill out: Section aA of Title United States, Applicant Signature Date, Note This application will be, Section Notary Acknowledgement, Instructions to Notary Modify, State or Commonwealth of County, ID with photograph ID, Type Identifying Number Expiration, Witness my hand and official seal, and Notary StampSeal.

Be very careful while filling in Type Identifying Number Expiration and Notary StampSeal, as this is the part where most users make mistakes.
Step 3: Go through the information you've entered into the blank fields and then press the "Done" button. After setting up a7-day free trial account here, you'll be able to download dea 252 form or send it via email promptly. The form will also be easily accessible in your personal account page with your each modification. If you use FormsPal, you can certainly complete forms without having to worry about personal data incidents or data entries being shared. Our protected software helps to ensure that your personal information is maintained safe.