With the help of the online PDF editor by FormsPal, you are able to fill in or change dea form 254 pdf here and now. To make our editor better and less complicated to utilize, we consistently work on new features, taking into consideration suggestions from our users. All it requires is a couple of easy steps:
Step 1: Access the PDF form in our editor by pressing the "Get Form Button" at the top of this page.
Step 2: With this advanced PDF editing tool, it is possible to accomplish more than simply fill out blank form fields. Express yourself and make your documents look faultless with customized textual content added in, or adjust the original content to perfection - all that comes along with an ability to add any type of images and sign the document off.
This form requires some specific information; in order to guarantee consistency, make sure you heed the guidelines listed below:
1. To start off, when filling out the dea form 254 pdf, beging with the section that has the next blanks:
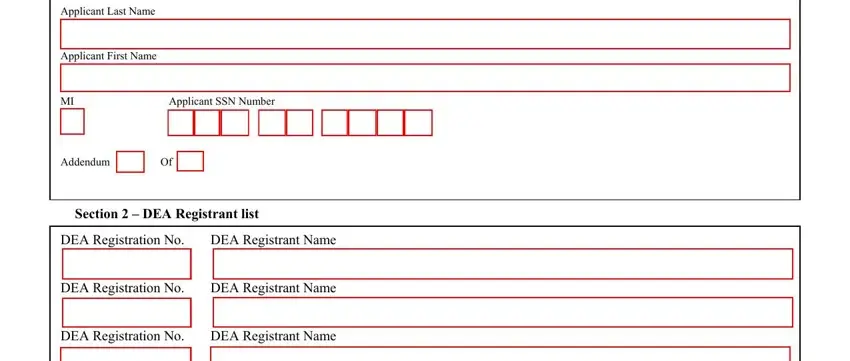
2. When the last segment is complete, it is time to put in the required specifics in DEA Registration No DEA, DEA Registrant Name, DEA Registrant Name, DEA Registrant Name, DEA Registrant Name, DEA Registrant Name, DEA Registrant Name, and DEA Registrant Name in order to move on further.
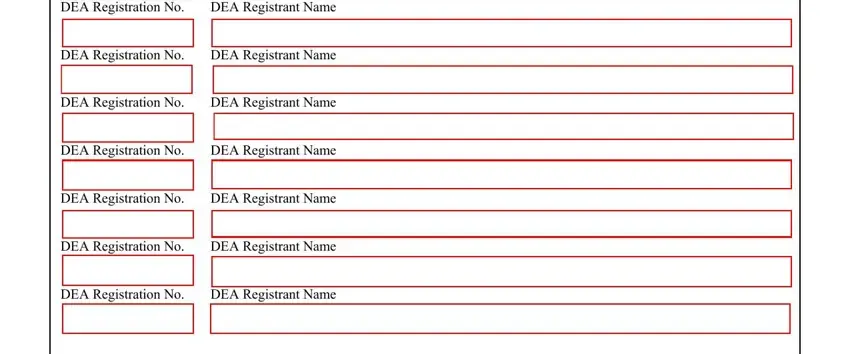
As to DEA Registrant Name and DEA Registrant Name, make certain you don't make any errors here. These could be the most important fields in this form.
Step 3: Prior to moving forward, make certain that blank fields are filled in properly. The moment you believe it is all good, click “Done." Sign up with FormsPal now and easily use dea form 254 pdf, set for downloading. Every last change you make is conveniently kept , which enables you to customize the pdf further as required. We do not sell or share the information you enter while filling out forms at our site.


