The State of California sets a high bar for regulatory compliance in the manufacturing of drug products, a benchmark embodied in the rigorously detailed CDPH 52N form provided by the California Department of Public Health's Food and Drug Branch. This form serves as a foundational pillar for entities aiming to secure a new drug manufacturing license, marking an essential step in navigating the complex regulatory landscape. With sections covering everything from the legal name of the firm, type of ownership, types of drug products manufactured, to manufacturing processes and fees, the form encapsulates a comprehensive snapshot of applicant operations. It is meticulously designed to gather pertinent information, including but not limited to, facility details, corporate structure, intended drug destinations, and manufacturing stages at the time of application. Each aspect of the form is structured to ensure that manufacturers meet the stringent standards set forth for drug manufacturing in California, reflecting a commitment to public health and safety. The inclusion of a detailed instruction section further underscores the state's dedication to clarity and compliance, guiding applicants through the process of submitting a fully completed application. This thorough approach facilitates a robust vetting process, ensuring that only qualified manufacturers can navigate the complexities of drug production within the state's jurisdiction.
| Question | Answer |
|---|---|
| Form Name | Form Cdph 52N |
| Form Length | 2 pages |
| Fillable? | No |
| Fillable fields | 0 |
| Avg. time to fill out | 30 sec |
| Other names | 24-Hour, PDMA, 52N, Rx |
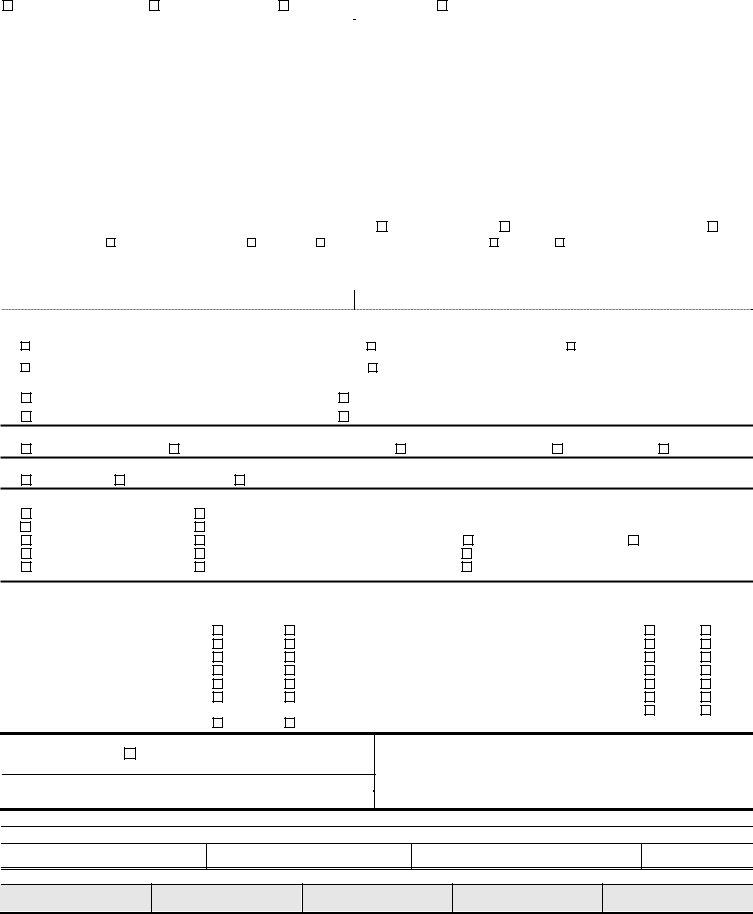
State of |
California Department of Public Health |
|
Food and Drug Branch |
NEW DRUG MANUFACTURING LICENSE APPLICATION
PLEASE COMPLETE THIS FORM
See Page 2 for Instructions
NEW APPLICANT
RELOCATION
OWNERSHIP CHANGE
OWNERSHIP AND LOCATION CHANGE
1. |
Legal Name of Firm |
|
|
|
|
|
9. |
Facility Operator (name and title) |
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
||||
2. |
DBA (List additional DBAs on separate sheet if necessary.) |
|
10. |
Facility Telephone Number |
|
11. |
Facility FAX Number |
|
||||||
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
||||
3. |
Facility Address (number, street) |
|
|
|
12. |
13. |
|
|||||||
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
4. |
Facility Address (continued) |
|
|
|
14. |
Correspondent (name and title) |
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|||
5. |
City |
|
State |
|
ZIP Code |
|
15. |
Correspondent Telephone Number |
16. |
Correspondent FAX Number |
|
|||
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
6. |
Mailing Address (if different or P.O. Box number) |
|
|
|
17. |
Country (if other than United States) |
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||
7. |
Mailing Address (continued) |
|
|
|
18. |
Website (URL) |
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8. |
City |
|
State |
|
ZIP Code |
|
19. |
Interstate Commerce |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product Shipped |
Product or Raw Materials Received |
N/A |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|||
20. |
Type of Ownership: |
Individual/Sole Proprietorship |
Partnership |
Corporation/Limited Liability Company |
Nonprofit |
Other: |
|
|
||||||
|
|
|
|
|
|
(attach copy of Partnership agreement or Articles of Incorporation) |
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
||||
21. |
Corporate Name (if applicable) |
|
|
|
State of Incorporation |
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
22. Owners’ or Officers’ Names and Titles
Owners’ or Officers’ Names and Titles (Attach separate sheet if necessary)
|
|
|
23. Size of Facility (square feet):________________ |
|
|
Business days and hours:____________________ |
Business License Number:_____________ |
Seller’s Permit:________________ |
Number of Employees at this Facility:____________________ |
Federal Employee Identification Number:__________________ |
|
24. Stage of Manufacture at Date of Application (check all that apply) |
|
|
Manufacturing products
Validation – Completion Date: _______________________
Plant construction/design (Targeted Completion Date: ____________________)
Other (specify): ____________________________________________________
25. Intended Drug Destination (check all that apply)
Commercial distribution
Human clinical trials (investigational use)
California distribution only
U.S. distribution
Export market
26. Type of Drug Product (check all that apply)
Prescription* |
|
*If Prescription or Both is checked complete the Disclosure Statement form (CDPH53) |
Both* |
refer to PDMA requirements on instruction page 2. |
27. Drug Products Manufactured at this Location (check all that apply)
700 Bulk pharmaceuticals (API)
708 Biologics
701 Medical gases
702Radioactive
703Veterinary
704Controlled substances (schedule: _______________DEA#:_____________(attach copy of DEA certificate)
712Topical
705 |
Approved New Drug |
709 Parenteral |
Other (specify): |
|
706 |
Investigational New Drugs (IND) |
710 |
Oral Dose (solid/liquid) |
__________________ |
707 |
Biotech |
711 |
|
|
28.Manufacturing processes/activities employed or planned in the manufacture of the drugs listed above. Indicate if these processes/activities will be done at this location
Processes/Activities |
Contract |
Processes/Activities |
||
Aerosolization |
|
|
Powder Mixing |
|
Aseptic |
|
|
Relabel Only |
|
Coating |
|
|
Repackage Only |
|
Emulsification |
|
|
Sterilization |
|
Encapsulation |
|
|
Suspension |
|
Fermentation/tissue culture viral |
|
|
Tableting |
|
vector/gene therapy |
|
|
Other (Specify):__________________ |
|
Liquid Mixing |
|
|
|
|
29. Payment Code |
MAKE CHECKS PAYABLE TO: CA DEPT. OF PUBLIC HEALTH
See page 2 for mailing address
30. License Fees Due: |
Enter Each Fee Below: |
a. License Fee (see #30) |
$ |
|
|
b. PDMA* ($100 If Applicable – see page 2) |
$ |
c. Total Payment Due |
$ |
The Food and Drug Branch MUST BE NOTIFIED of any change in the application information as provided by CA Health and Safety Code, §111630. By signature, I declare under penalty of perjury that all information provided herein is true and correct.
31. Signature
Printed Name
Title
Date
PLEASE DO NOT WRITE BELOW THIS LINE.
License Number
Expiration Date
Date Received
Payment Type
Amount
$
CDPH 52N (06/09) |
Fund 3018 Index 5623 PCA 76213 Receipt Source 125700 Agency Source 44 |
Page 1 of 2 |
NEW DRUG MANUFACTURING LICENSE APPLICATION INSTRUCTIONS
A separate application is required for each place of business. Please complete and/or amend this application as is most appropriate to your facility. Include the appropriate fee for each application and make payable to: CA DEPARTMENT OF PUBLIC HEALTH. The fee must accompany this application or it cannot be processed. Unsigned or incomplete applications cannot be processed. The following are further instructions on how to complete this application:
New Applicant: Place an (X) in the box next to New Applicant if your firm has not previously applied for a Drug Manufacturing License at this location while under the current ownership. This license is
1.Name of Firm: Enter full name of business, corporation, company, or organization applying for licensure.
2.DBA: Enter any other name(s) your company is doing business as.
9. |
Facility Operator: Enter the full name(s) of the person(s) in charge of drug manufacturing at this facility and their title(s). |
|||||||
10. |
Facility Telephone Number: Enter daytime business telephone number of this facility. |
|
|
|||||
11. |
Facility FAX Number: Enter facility FAX number. |
|
|
|
|
|
|
|
12. |
|
|
||||||
13. |
|
|
|
|
|
|
||
14. |
Correspondent: Enter the name of the person to contact for information regarding this application and their title. |
|
|
|||||
15. |
Correspondent Telephone Number: Enter the daytime business telephone number of the contact person. |
|
|
|||||
16. |
Correspondent FAX Number: Enter the daytime business FAX number of the contact person. |
|
|
|||||
17. |
Country: Enter the country where your facility is located, if outside of the United States. |
|
|
|||||
18. |
Website: Enter the website address for your business, if applicable. |
|
|
|
|
|||
19. |
Interstate Commerce: Place an (X) in all appropriate boxes that correctly describe your business’ receipt or distribution of products or materials |
|||||||
|
through or into interstate commerce. |
|
|
|
|
|
|
|
20. |
Type of Ownership: Place an (X) in the box next to the appropriate legal description of the facility’s ownership. |
|
|
|||||
21. |
Corporate Name: Enter corporate name if applicable. Enter state of incorporation if applicable. (Attach copy) |
|
|
|||||
22. |
Owners’ or Officers’ Names: List the business owners’ or officers’ names and titles. USE ADDITIONAL SHEETS IF NECESSARY. |
|||||||
23. |
Size of Facility: Indicate the approximate size (in square feet) of the facility and the approximate number of employees at the facility and list |
|||||||
|
business days and hours. Enter the Business license, FEIN, and Seller’s Permit and provide required copies. |
|
|
|||||
24. |
Stage of Manufacture: Place an (X) in the box next to the stage of manufacture your products are in at the time of application submission. Check |
|||||||
|
all that apply. |
|
|
|
|
|
|
|
25. |
Intended Drug Destination: Place an (X) in the box adjacent to the destination(s) for your manufactured products. Check all that apply. |
|||||||
26. |
Types of Products: Place an (X) in each box that applies to each type of drugs manufactured or to be manufactured. For human prescription (Rx) |
|||||||
|
drug manufacturers, refer to PDMA requirements below*. |
|
|
|
|
|
||
27. |
Products Manufactured: Place an (X) in the box adjacent to each product area that applies to the drugs manufactured or to be manufactured. Use |
|||||||
|
additional sheets if necessary. (attach copy of DEA certificate) |
|
|
|
|
|
||
28. |
Manufacturing Processes: Place an (X) in the columns adjacent to all applicable processes to be performed |
|||||||
|
Leave line blank if the indicated process will not be applied to the manufacturing of listed drugs. List additional processes or methods as needed |
|||||||
|
herein or on additional sheets if necessary. |
|
|
|
|
|
|
|
29. |
Payment Fee Code: Your license fee is based on the application type **. |
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
Application Type |
|
Fee |
|
Payment Interval |
|
Payment Code |
|
|
|
|
|
|
|
|
|
|
|
New, Relocation, or Ownership Change |
|
$1600 |
First License only |
|
A |
|
|
|
|
|
|
|
|
|
||
|
* PDMA (Prescription Drug Marketing Act) Requirements: |
If your firm manufactures human prescription (Rx) drugs, an |
||||||
|
additional $100 must be added to the license fee and a Disclosure Statement (Form CDPH 53) must be submitted for each person |
|||||||
|
listed on lines #9 and #23 (instructions provided therein). Information relevant to the PDMA, (e.g., Disclosure Statements and |
|||||||
|
Applicant Fingerprint Live Scan requirements) can be reviewed at: http://www.cdph.ca.gov/fdb/HTML/Drug/PDMA.htm. |
|||||||
** |
LICENSE FEES ARE |
|||||||
30. |
License Fee Due: Enter appropriate fees due. |
|
|
|
|
|
|
|
|
a. Enter license fee according to payment codes in #30. (License valid for 1 year.) |
|
|
|||||
|
b. Add $100 PDMA fee if it applies to your firm. See PDMA requirements above*. |
|
|
|||||
|
c. Enter Total Payment Due by adding ”A” and “B” |
|
|
|
|
|
|
|
31. |
Sign the application, print your name, print your title, and enter the date. All signatures must be original. |
|
|
|||||
|
Make checks payable to: CA DEPARTMENT OF PUBLIC HEALTH |
Mail Application and Check to: |
|
|
||||
Regular Mail: California Department of Public Health |
|
Overnight Mail: California Department of Public Health |
||||||
|
Food and Drug Branch - Cashier |
|
|
Food and Drug Branch - Cashier |
||||
|
MS 7602 |
|
|
1500 Capitol Avenue, |
||||
|
P.O. Box 997435 |
|
|
Sacramento, CA 95814 |
||||
|
Sacramento, CA |
|
|
|
|
|
|
|
If you have any questions about this application, please contact the FDB License Desk for Drug Manufacturing at (916)
CDPH 52N (06/09) |
Fund 3018 Index 5623 PCA 76213 Receipt Source 125700 Agency Source 44 |
Page 2 of 2 |