State of California—Health and Human Services Agency |
|
|
|
|
|
|
|
|
|
California Department of Public Health |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Food and Drug Branch |
|
BIENNIAL MEDICAL DEVICE MANUFACTURING LICENSE RENEWAL APPLICATION |
|
PLEASE COMPLETE THIS FORM FULLY—INCOMPLETE APPLICATIONS WILL BE RETURNED |
|
|
|
|
|
|
See page 2 for instructions |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
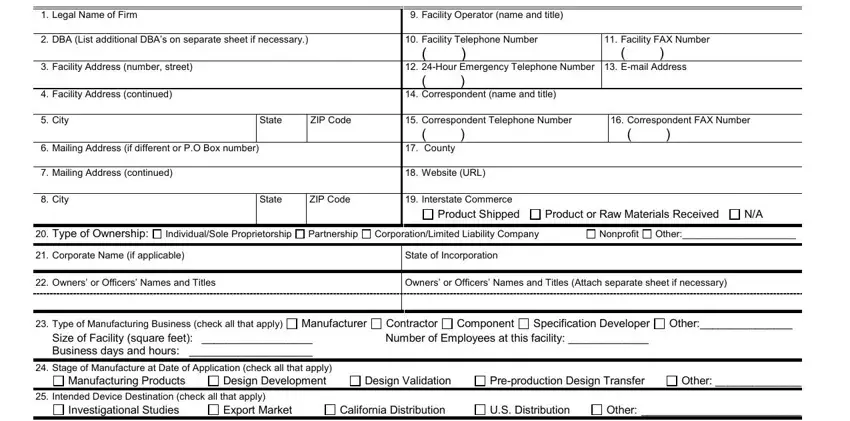
1. |
Legal Name of Firm |
|
|
|
|
9. |
Facility Operator (name and title) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2. |
DBA (List additional DBA’s on separate sheet if necessary.) |
|
|
10. |
Facility Telephone Number |
|
|
11. Facility FAX Number |
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
3. |
Facility Address (number, street) |
|
|
12. |
24-Hour Emergency Telephone Number |
|
13. E-mail Address |
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
4. |
Facility Address (continued) |
|
|
14. |
Correspondent (name and title) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5. |
City |
|
State |
ZIP Code |
|
15. |
Correspondent Telephone Number |
|
|
16. Correspondent FAX Number |
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
6. |
Mailing Address (if different or P.O Box number) |
|
|
17. |
County |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7. |
Mailing Address (continued) |
|
|
18. |
Website (URL) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8. |
City |
|
State |
ZIP Code |
|
19. |
Interstate Commerce |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product Shipped |
Product or Raw Materials Received |
N/A |
|
|
|
|
|
|
|
|
|
|
|
20. |
Type of Ownership: |
Individual/Sole Proprietorship |
Partnership |
Corporation/Limited Liability Company |
|
Nonprofit |
Other:_____________________ |
|
|
|
|
|
|
|
|
|
|
|
|
21. |
Corporate Name (if applicable) |
|
|
State of Incorporation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
22. |
Owners’ or Officers’ Names and Titles |
|
|
Owners’ or Officers’ Names and Titles (Attach separate sheet if necessary) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
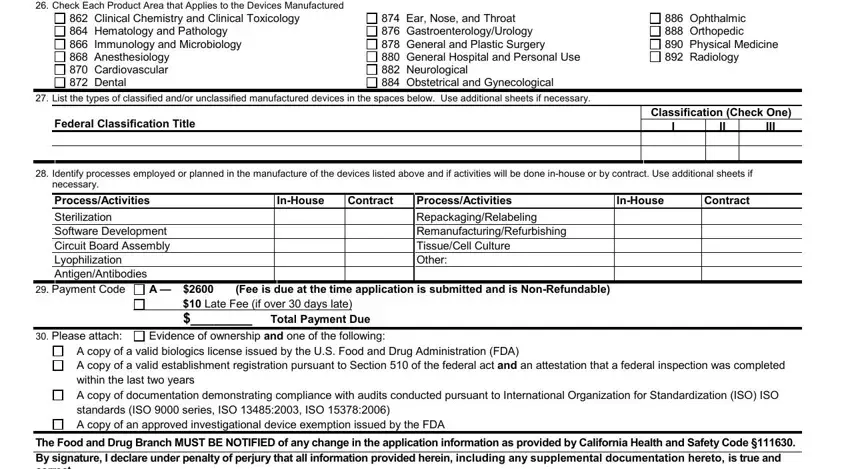
23.Type of Manufacturing Business (check all that apply) Manufacturer Size of Facility (square feet): __________________
Business days and hours: ____________________
Contractor Component Specification Developer Number of Employees at this facility: _____________
24. Stage of Manufacture at Date of Application (check all that apply)
Manufacturing Products |
Design Development |
Pre-production Design Transfer
25. Intended Device Destination (check all that apply)
Investigational Studies |
Export Market |
Other: __________________________
26. Check Each Product Area that Applies to the Devices Manufactured
862Clinical Chemistry and Clinical Toxicology
864Hematology and Pathology
866Immunology and Microbiology
868Anesthesiology
870Cardiovascular
872Dental
874Ear, Nose, and Throat
876Gastroenterology/Urology
878General and Plastic Surgery
880General Hospital and Personal Use
882Neurological
884Obstetrical and Gynecological
886Ophthalmic
888Orthopedic
890Physical Medicine
892Radiology
27. List the types of classified and/or unclassified manufactured devices in the spaces below. Use additional sheets if necessary.
Federal Classification Title
Classification (Check One)
28.Identify processes employed or planned in the manufacture of the devices listed above and if activities will be done in-house or by contract. Use additional sheets if necessary.
|
Process/Activities |
|
|
In-House |
Contract |
Process/Activities |
In-House |
Contract |
|
Sterilization |
|
|
|
|
|
|
Repackaging/Relabeling |
|
|
|
Software Development |
|
|
|
|
|
Remanufacturing/Refurbishing |
|
|
|
Circuit Board Assembly |
|
|
|
|
|
Tissue/Cell Culture |
|
|
|
Lyophilization |
|
|
|
|
|
|
Other: |
|
|
|
Antigen/Antibodies |
|
|
|
|
|
|
|
|
|
29. Payment Code |
A — |
$2600 |
(Fee is due at the time application is submitted and is Non-Refundable) |
|
|
|
|
|
|
|
$10 Late Fee (if over 30 days late) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$________ Total Payment Due |
|
|
|
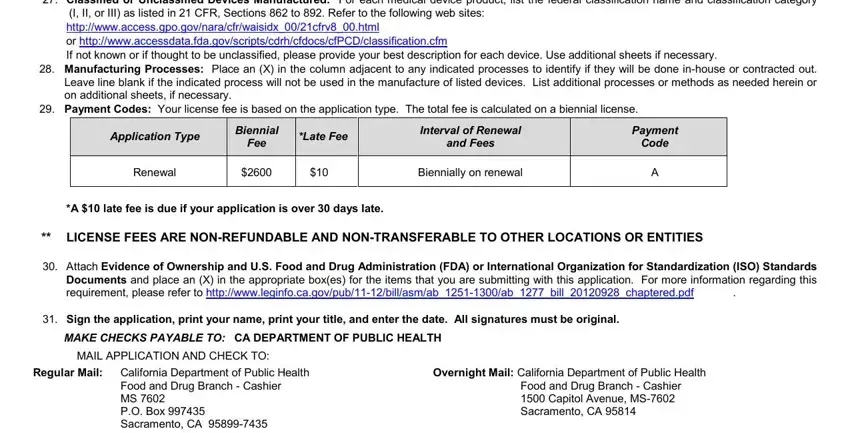
30. Please attach: |
Evidence of ownership and one of the following: |
|
|
|
A copy of a valid biologics license issued by the U.S. Food and Drug Administration (FDA)
A copy of a valid establishment registration pursuant to Section 510 of the federal act and an attestation that a federal inspection was completed within the last two years
A copy of documentation demonstrating compliance with audits conducted pursuant to International Organization for Standardization (ISO) ISO standards (ISO 9000 series, ISO 13485:2003, ISO 15378:2006)
A copy of an approved investigational device exemption issued by the FDA
The Food and Drug Branch MUST BE NOTIFIED of any change in the application information as provided by California Health and Safety Code §111630.
By signature, I declare under penalty of perjury that all information provided herein, including any supplemental documentation hereto, is true and correct.
31. Signature of Applicant
PLEASE DO NOT WRITE BELOW THIS LINE.
CDPH 72R (01/13) |
Fund 3018 Index 5624 PCA 76211 Receipt Source 125700 Agency Source 0049 |
Page 1 of 2 |
Biennial Medical Device Manufacturing License Renewal Application Instructions
A separate application is required for each place of business. Please complete and/or amend this application as is most appropriate to your facility. Include the appropriate fee for each application as indicated in the fee schedule and make payable to: CA DEPARTMENT OF PUBLIC HEALTH. This fee must accompany this application or the application cannot be processed. Please apply within 30 days of expiration; failing to do so requires an additional $10 penalty added to the renewal fee before the license is issued. Unsigned or incomplete applications cannot be processed. The following are further instructions on how to complete this application
Renewal Applicant: This license is non-transferable. If your firm has changed location, ownership, or both, use the application titled “New Medical Device Manufacturing License Application” (CDPH 72N). Any questions that do not apply to your company indicate with N/A. Do not leave any sections blank.
1.Legal Name of Firm: Enter full legal name of business, corporation, company, or organization applying for licensure.
2.DBA: Enter any other name(s) your company is doing business as.
3.–5. Facility Address: Enter the number, street, city, state, and ZIP code for this facility location.
6.–8. Mailing Address: Enter the full mailing address if different from the facility address or P.O Box.
9.Facility Operator: Enter the full name of the person who is responsible for the manufacturing of medical devices at this facility and their title.
10.Facility Telephone Number: Enter daytime business telephone number of this facility.
11.Facility FAX Number: Enter facility FAX number.
12.24-Hour Emergency Telephone Number: Enter telephone number to be called in the event of an emergency.
13.E-mail Address: Enter facility or correspondent’s email address.
14.Correspondent: Enter the name of the person to contact for information regarding this application and their title.
15.Correspondent Telephone Number: Enter the daytime business telephone number of the contact person.
16.Correspondent FAX Number: Enter the daytime business FAX number of the contact person.
17.County: Enter the county where your facility is located.
18.Website: Enter the website address for your business.
19.Interstate Commerce: Place an (X) in the boxes that correctly describe your business’ receipt or distribution of products or materials through or into interstate commerce.
20.Type of Ownership: Place an (X) in the box next to the appropriate legal description of the facility’s ownership.
21.Corporate Name: Enter corporate name if applicable. Enter the state of incorporation if applicable.
22.Owner’s or Officer’s Names: List the business owners’ or officers’ names and titles.
23.Type of Manufacturing Business: Place an (X) in the box next to each type of manufacturing business conducted at this facility, size of facility, number of employees, and list business days and hours.
24.Stage of Manufacture: Place an (X) in the box next to the stage of manufacture your products are in at the time of application submission. Check all that apply.
25.Intended Device Destination: Place an (X) in the box adjacent to the destination(s) for your manufactured products. Check all that apply.
26.Products Manufactured: Place an (X) in the box adjacent to each product area that applies to the devices manufactured or to be manufactured.
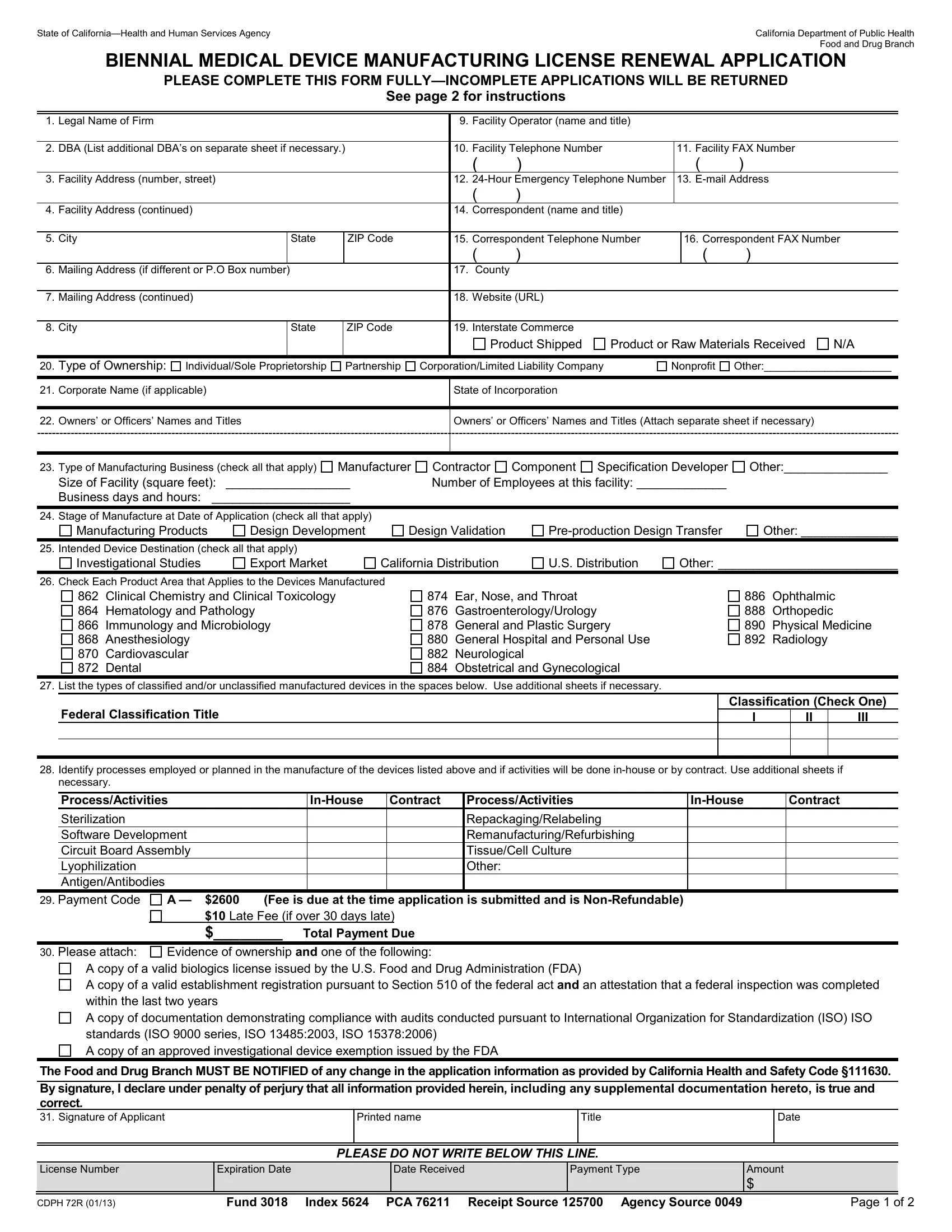
27.Classified or Unclassified Devices Manufactured: For each medical device product, list the federal classification name and classification category (I, II, or III) as listed in 21 CFR, Sections 862 to 892. Refer to the following web sites: http://www.access.gpo.gov/nara/cfr/waisidx_00/21cfrv8_00.html
or http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPCD/classification.cfm
If not known or if thought to be unclassified, please provide your best description for each device. Use additional sheets if necessary.
28.Manufacturing Processes: Place an (X) in the column adjacent to any indicated processes to identify if they will be done in-house or contracted out. Leave line blank if the indicated process will not be used in the manufacture of listed devices. List additional processes or methods as needed herein or on additional sheets, if necessary.
29.Payment Codes: Your license fee is based on the application type. The total fee is calculated on a biennial license.
|
|
|
|
Biennial |
|
|
|
|
|
Interval of Renewal |
|
|
Payment |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Application Type |
|
* |
Late Fee |
|
|
|
|
Fee |
|
|
|
and Fees |
|
|
Code |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Renewal |
$2600 |
|
$10 |
|
|
Biennially on renewal |
|
A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
*A $10 late fee is due if your application is over 30 days late.
**LICENSE FEES ARE NON-REFUNDABLE AND NON-TRANSFERABLE TO OTHER LOCATIONS OR ENTITIES
30.Attach Evidence of Ownership and U.S. Food and Drug Administration (FDA) or International Organization for Standardization (ISO) Standards Documents and place an (X) in the appropriate box(es) for the items that you are submitting with this application. For more information regarding this
requirement, please refer to http://www.leginfo.ca.gov/pub/11-12/bill/asm/ab_1251-1300/ab_1277_bill_20120928_chaptered.pdf |
. |
31.Sign the application, print your name, print your title, and enter the date. All signatures must be original.
MAKE CHECKS PAYABLE TO: CA DEPARTMENT OF PUBLIC HEALTH
MAIL APPLICATION AND CHECK TO: |
|
Regular Mail: California Department of Public Health |
Overnight Mail: California Department of Public Health |
Food and Drug Branch - Cashier |
Food and Drug Branch - Cashier |
MS 7602 |
1500 Capitol Avenue, MS-7602 |
P.O. Box 997435 |
Sacramento, CA 95814 |
Sacramento, CA 95899-7435 |
|
If you have any questions about this application, please contact the Food and Drug Branch, Medical Device Manufacturing Licensing Desk at (916) 650-6500 or by email at FDBMedDevice@cdph.ca.gov, or visit our web site at: http://www.cdph.ca.gov/programs/Pages/FDB.aspx.
CDPH 72R (01/13) |
Fund 3018 Index 5624 PCA 76211 Receipt Source 125700 Agency Source 0049 |
Page 2 of 2 |