Filling out the Form Dea 106 form is easy with this PDF editor. Stick to the next steps to create the document in a short time.
Step 1: The first step is to click on the orange "Get Form Now" button.
Step 2: After you've accessed the Form Dea 106 editing page you'll be able to discover the different functions you'll be able to carry out regarding your document from the upper menu.
To prepare the Form Dea 106 PDF, enter the information for all of the segments:

Put down the details in the No Have there been losses in, Yes If yes how many excluding this, Yes, Name of Consignee Supplier Enter, DEA Registration Number of, If this was a robbery were any, Yes If yes how many Were any, Yes If yes how many, What is the total value of the, This is the amount you paid for, Was theft reported to Police, Yes If yes fill out the following, Name of Police Department Police, Name of Responding Officer Phone, and Which corrective measures have field.

The software will request you to include certain necessary data to instantly submit the field Which corrective measures have, Installed monitoring equipment eg, Provided security training to, Were any pharmaceuticals or, Yes Estimated Value, and Form DEA Pg.

Through box s e l p m a x E, Demerol, Robitussin AC, Meperidine Hydrochloride, Codeine Phosphate, mgml, mgcc, Vial, and Liquid, state the rights and obligations.
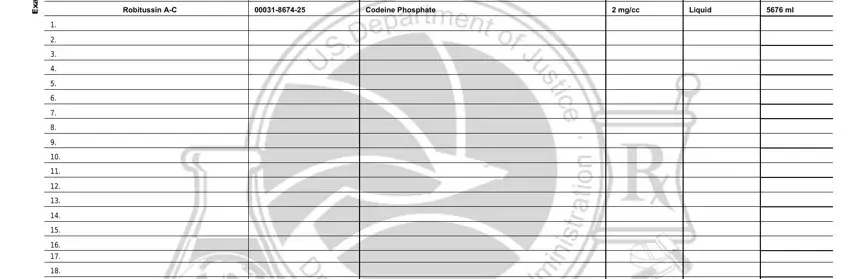
End by reviewing all these fields and filling them in as required: Remarks Optional, Form DEA Pg, and Express Quantity in Dosage Units.

Step 3: Click the button "Done". The PDF form is available to be exported. It is possible to upload it to your laptop or email it.
Step 4: It's going to be more convenient to create duplicates of the form. There is no doubt that we won't disclose or read your particulars.

 No
No  Yes
Yes  No
No  Yes
Yes  Installed monitoring equipment (e.g. video camera).
Installed monitoring equipment (e.g. video camera). Increased employee monitoring (e.g. random drug tests).
Increased employee monitoring (e.g. random drug tests). Installed metal bars or other security on doors or windows.
Installed metal bars or other security on doors or windows. Secured Controlled Substances within safe.
Secured Controlled Substances within safe. Other (Please describe on last page).
Other (Please describe on last page).
 Provided security training to staff.
Provided security training to staff.
 Requested increased security patrols by Police.
Requested increased security patrols by Police. Hired security guards for premises.
Hired security guards for premises. 
 Terminated employee.
Terminated employee.


